Abstract.
Low-density malaria infections are a source of human morbidity in endemic settings and potentially contribute to ongoing malaria transmission. Conventional rapid diagnostic tests (RDTs) were designed to detect clinically relevant parasite and antigen levels, but it is largely unknown what proportion of parasite (and antigen positive) infections are missed by conventional RDTs. Furthermore, RDTs can also provide false positives from lingering histidine-rich protein 2 (HRP2) antigenemia from a past infection. We analyzed 207 samples from Angolan outpatients with a bead-based HRP2 antigen assay and by qRT-PCR for the presence of parasite nucleic acids. Among patients HRP2 positive but negative by conventional RDT, the rate of quantitative reverse transcription-PCR (qRT-PCR) positivity was 45% (95% CI: 35–56%), with a median parasitemia of 3.4 parasites/µL (interquartile range: 0.14–4.8). Only 15% (7–26%) of HRP2-negative samples were found to have parasite nucleic acids. A substantial proportion of persons with blood HRP2 antigen concentrations not detected by the conventional RDT were found to have evidence of active infection, but at low parasite density levels.
Detection of the Plasmodium falciparum antigen histidine-rich protein 2 (HRP2) is the basis for most malaria diagnosis worldwide, including in sub-Saharan Africa.1 However, conventional HRP2-based diagnostic tests remain positive for an extended period following successful P. falciparum parasite clearance because the antigen lingers weeks after resolution of infection.2 As a result, HRP2 antigen presence does not necessarily imply active parasite infection. This limitation is particularly relevant in the context of the recent development of field rapid diagnostic tests (RDTs) with higher sensitivity for the detection of HRP2 in blood samples.3 Recently, we reported that a conventional RDT used at health facilities in two Angolan provinces detected 81% and 82% of HRP2 antigenemias in febrile patients and 52% and 77% in afebrile patients, when compared with an ultrasensitive bead-based laboratory assay.4,5 However, because we lacked data on active infection status, we were unable to report the proportion of patient samples positive for HRP2 by the bead assay but negative by RDT that represented active P. falciparum infection.
To help clarify the utility of the HRP2 assay for laboratory detection of active infections, we used a qRT-PCR assay for ultrasensitive detection of Plasmodium 18S ribosomal RNA (rRNA) to analyze a subset of samples (16.4% of all samples) from Angolan outpatients collected during a health facility survey6 and compared the qRT-PCR results with the results of the HRP2-based diagnostic and laboratory tests. In total, 207 samples were selected to include a range of antigen levels, including 61 negative by RDT (SD Bioline P. falciparum/Plasmodium vivax, Standard Diagnostics, Yongin, Republic of Korea) and negative by the bead-based HRP2 assay (RDT−/HRP2−); 93 negative by RDT but positive by the bead-based HRP2 assay (RDT−/HRP2+); and 53 samples positive by RDT (RDT+), 51 of which were also positive by the bead-based HRP2 assay. We extracted total nucleic acids (DNA Mini Kit, Qiagen, Hilden, Germany) and quantitatively amplified 18S rRNA using pan-Plasmodium primers and probes on the Abbott m2000 sp/rt system (Abbott, Chicago, IL) as described previously.7–9 Parasite densities were estimated using a conversion factor of 7.4 × 103 copies per ring-stage P. falciparum parasite,7 and the level of detection of the qRT-PCR assay corresponded to 0.020 parasites/µL of whole blood. Additional analysis of the anonymous blood samples from the Angola survey was approved by the Office of the Associate Director for Science, Center for Global Health at CDC as research, not involving human subjects (2018-034).
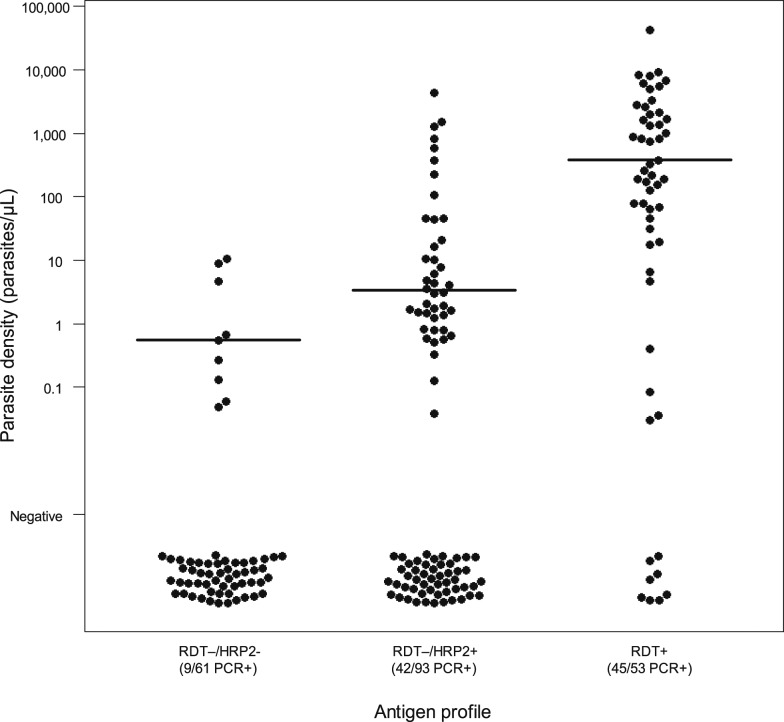
Nine of 61 RDT−/HRP2− samples were positive by qRT-PCR for P. falciparum rRNA (15%, 95% CI: 7–26%) (Figure 1). Forty-two of 93 samples were qRT-PCR+ (45%, 95% CI: 35–56%) from persons HRP2+ but where the individual had provided a negative RDT result (RDT−/HRP2+). By contrast, 45 of 53 RDT+ samples were qRT-PCR positive (85%, 95% CI: 72–93%). Estimated parasite density in samples qRT-PCR positive followed a similar pattern, with a median of 0.56 parasites/µL (interquartile range [IQR]: 0.14–4.8) in the RDT−/HRP2− samples, 3.4 parasites/µL (IQR: 1.3–39) in the RDT−/HRP2+ category, and 377 parasites/µL (IQR: 70–2,161) in the RDT+ samples (Figure 1). Rates of qRT-PCR positivity (chi-squared P-value < 0.01) and the distribution of parasite densities (all pairwise Kolmogorov–Smirnov P-values < 0.05) differed significantly among the three categories.
Figure 1.
Distribution of malaria parasite density, as assessed by qRT-PCR, for samples from Angolan outpatients grouped by conventional RDT and ultrasensitive bead-based HRP2 laboratory assay result. HRP2 = histidine-rich protein 2; RDT = rapid diagnostic test.
The samples without detectable HRP2 (even with the ultrasensitive bead-based assay) but positive by qRT-PCR could represent infections where the blood stage parasite is present at significant levels but with unusually low expression of the antigen or where the parasite is present at very low numbers, such as in the case of an early infection where not enough HRP2 has accumulated. Previous estimates showed the HRP2 bead assay reliably detected the HRP2 antigen in P. falciparum parasite densities approaching 1 parasite/µL, but lost sensitivity at densities lower than this.5 To this point, the level of estimated parasite density for those qRT-PCR positive in this HRP2− category was very low, with most infections less than 1.0 parasites/µL. Data on the contribution of infections with such a low parasite density to ongoing malaria transmission are scarce and conflicting.10,11 Nearly half (45%) of all samples with laboratory-detectable HRP2 but negative RDTs showed molecular evidence of active P. falciparum infection. The remaining 55% qRT-PCR–negative samples in this category likely represents past infections but with HRP2 levels lower than what is detected by the RDT, but high enough to be detected by the bead assay. Conversely, the eight (15%) samples from RDT-positive persons that were not found to contain any P. falciparum nucleic acids suggest either a false-negative qRT-PCR result, false-positive RDT, or lingering HRP2 antigen2 from previous (not current) parasite infections for these individuals. Further studies using highly sensitive laboratory diagnostic methods, such as the bead-based assay and qRT-PCR, will build the evidence base on the relationship between infection status and the results of malaria diagnostic tests and will help to inform the interpretation of the epidemiological significance of these tests.
Acknowledgments:
We acknowledge Venkatachalam Udhayakumar for his assistance in manuscript preparation. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention. M. M. P. and E. S. H. were supported by the U.S. President’s Malaria Initiative. We declare that we do not have any commercial or other associations that might pose a conflict of interest.
REFERENCES
- 1.WHO , 2017. World Malaria Report 2017. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 2.Plucinski M, et al. 2017. Post-treatment HRP2 clearance in patients with uncomplicated Plasmodium falciparum malaria. J Infect Dis 217: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Das S, et al. 2017. Performance of a high-sensitivity rapid diagnostic test for Plasmodium falciparum malaria in asymptomatic individuals from Uganda and Myanmar and naive human challenge infections. Am J Trop Med Hyg 97: 1540–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plucinski M, Rogier E, Dimbu PR, Fortes F, Halsey ES, Aidoo M, 2017. Estimating the added utility of highly sensitive HRP2 detection in outpatient clinics in sub-saharan Africa. Am J Trop Med Hyg 97: 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rogier E, et al. 2017. Bead-based immunoassay allows sub-picogram detection of histidine-rich protein 2 from Plasmodium falciparum and estimates reliability of malaria rapid diagnostic tests. PLoS One 12: e0172139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Plucinski MM, et al. 2017. Evaluating malaria case management at public health facilities in two provinces in Angola. Malar J 16: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murphy SC, Prentice JL, Williamson K, Wallis CK, Fang FC, Fried M, Pinzon C, Wang R, Talley AK, Kappe SH, 2012. Real-time quantitative reverse transcription PCR for monitoring of blood-stage Plasmodium falciparum infections in malaria human challenge trials. Am J Trop Med Hyg 86: 383–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Billman ZP, Seilie AM, Murphy SC, 2016. Purification of Plasmodium sporozoites enhances parasite-specific CD8+ T cell responses. Infect Immun 84: 2233–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Murphy SC, Daza G, Chang M, Coombs R, 2012. Laser cutting eliminates nucleic acid cross-contamination in dried-blood-spot processing. J Clin Microbiol 50: 4128–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouédraogo AL, Gonçalves BP, Gnémé A, Wenger EA, Guelbeogo MW, Ouédraogo A, Gerardin J, Bever CA, Lyons H, Pitroipa X, 2015. Dynamics of the human infectious reservoir for malaria determined by mosquito feeding assays and ultrasensitive malaria diagnosis in Burkina Faso. J Infect Dis 213: 90–99. [DOI] [PubMed] [Google Scholar]
- 11.Lin JT, Saunders DL, Meshnick SR, 2014. The role of submicroscopic parasitemia in malaria transmission: what is the evidence? Trends Parasitol 30: 183–190. [DOI] [PMC free article] [PubMed] [Google Scholar]