Figure 1.
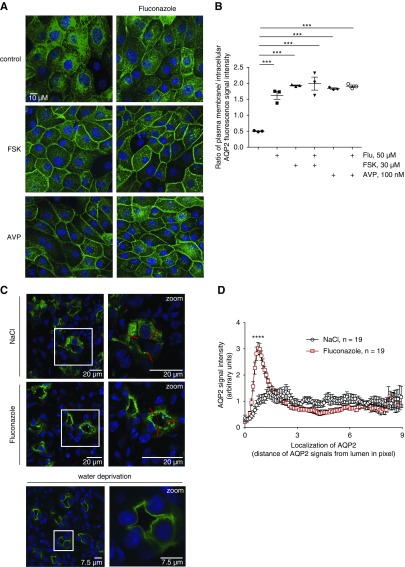
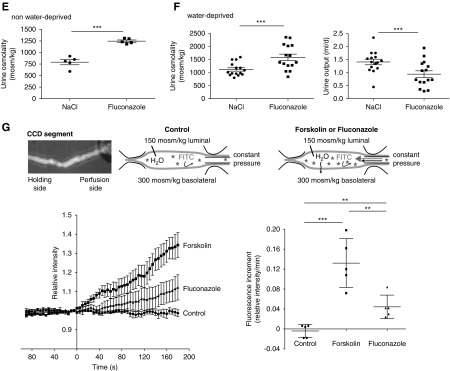
Fluconazole (Flu) promotes a plasma membrane localization of aquaporin-2 (AQP2), and it decreases urine output and increases urine osmolality in mice. (A) Primary inner medullary collecting duct cells were left untreated (control) or stimulated with forskolin (FSK; 30 μM, 30 minutes), arginine-vasopressin (AVP; 100 nM, 30 minutes), or Flu (50 μM, 60 minutes) alone or in combination with FSK or AVP (addition of FSK or AVP for an additional 30 minutes). AQP2 was detected by immunofluorescence microscopy using specific primary (H27) and Cy3-coupled secondary antibodies (green). Nuclei were stained with 4′,6-diamidine-2′-phenylindole dihydrochloride (blue). Shown are representative images from one of three independent experiments. (B) Intensities of intracellular and plasma membrane immunofluorescence signals arising from AQP2 were determined, related to perinuclear signal intensities, and ratios of plasma membrane to intracellular signal intensities were calculated. Ratios less than one indicate a predominant intracellular localization, and ratios greater than one indicate a predominant plasma membrane location of AQP2 (mean±SEM; n=3 independent experiments). ***P<0.001. (C–F) Wild-type C57bl/6N male mice were treated every 24 hours over a period of 96 hours with Flu (intraperitoneal injections; 80 mg/kg body wt) or 0.9% saline (NaCl), or they were water deprived without additional treatment. Schemes for depicting animal treatments are indicated in Supplemental Figure 1. (C) Tissue sections from kidneys were prepared, and AQP2 and nuclei were detected as indicated in A. Shown are representative images from three independent experiments each carried out with five animals per condition. The magnified views of collecting ducts are from the indicated white boxes. (D) The AQP2 localization was semiquantitatively analyzed in three animals (n=19 collecting ducts of each Flu and NaCl treated). Plotted is the AQP2 signal intensity along the indicated red line. The peak in Flu signals corresponds to the apical plasma membrane of collecting duct principal cells (mean±SEM). ****P<0.001. (E) Baseline urine osmolality measurements with mice that had access to water ad libitum were carried out in stock cages using spot urine. (F) Water-deprived animals were kept in metabolic cages during the final 24 hours of the experiment. Urine was collected, and urine osmolality and output were monitored. n=15 animals per water-derived group and n=5 per nonwater-deprived group. Statistically significant differences are indicated (mean±SEM). ***P<0.001. (G) In vitro microperfusion of cortical collecting ducts (CCDs) isolated from wild-type C57Bl/6J mice. (Upper left panel) Combined light microscopy and fluorescence (485-nm) image of a live CCD segment in perfusion with 150 kD FITC dextran in the lumen. The CCD segments were occluded at the distal end with a holding pipette and connected on the opposite side to a perfusion pipette. (Upper center panel) The experimental setting in the control situation in the absence of treatment and on DMSO application. (Upper right panel) In the presence of FSK or Flu, water exits the lumen and is replenished via the perfusion pipette, which leads to a continuous increase of intraluminal FITC dextran. An osmotic gradient is established through the presence of 150 mosm/kg solution within the CCD lumen and 300 mosm/kg solution in the bath (basolateral). The CCDs were perfused at a constant pressure, and continuous monitoring of the fluorescence emission intensity from the lumen was started. On reaching a stable baseline of at least 90 seconds, FSK (30 μM), Flu (50 μM), or DMSO as a control (0.1%) was added (time point: 0 seconds). Fluorescence was measured for another 180 seconds. Results are summarized in the lower left panel. Increased water flow across the epithelia was quantitatively expressed as fluorescence increment values as a direct measure of water permeation velocity (lower right panel). Statistically significant differences are indicated (mean±SEM). For control, n=5 CCDs from five animals. For FSK, n=5 CCDs from five animals, and for Flu, n=5 CCDs from four animals. Each CCD was measured independently. ***P<0.001.