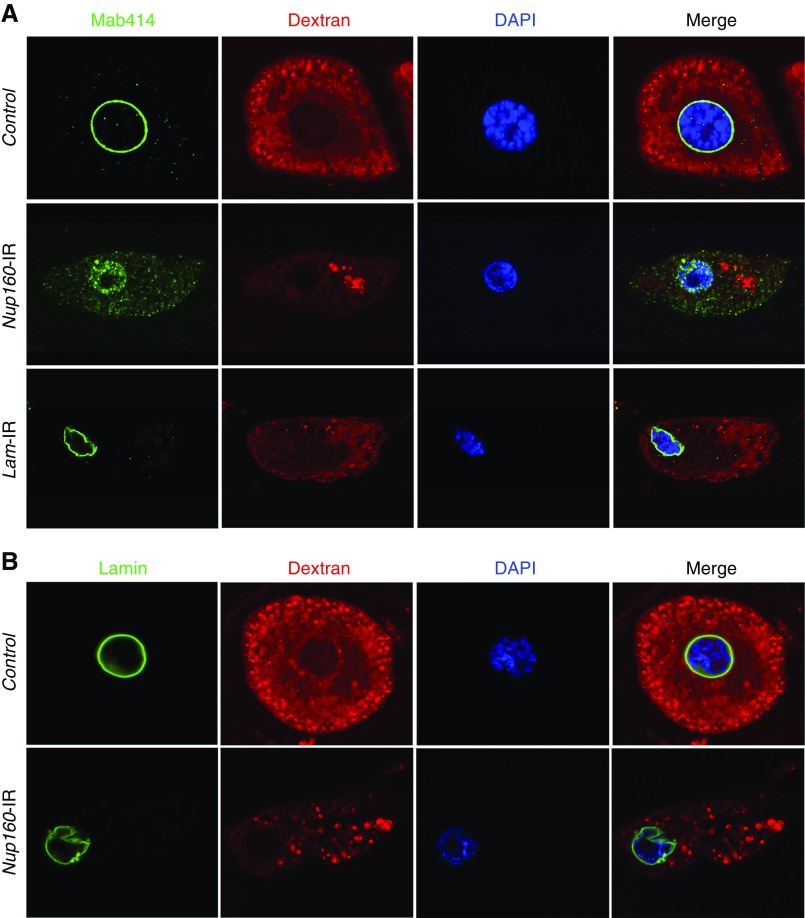
Figure 4.
Nup160 gene silencing induced nuclear pore complex (NPC) and lamin abnormalities in Drosophila nephrocytes. (A) Mab414 (green) immunolabeled components of the NPC, which in control nephrocytes, generated a sharp and highly regular ring around the nucleus (indicated by 4′,6-diamidino-2-phenylindole [DAPI] staining; blue). In nephrocytes of larvae in which Nup160 gene expression was silenced (Nup160-IR), by contrast, Mab414 immunolabeling was dispersed across the nucleus, and it was more abundant in the cytoplasm, indicating abnormal localization of NPC components. Normal ex vivo uptake of FITC-labeled 10-kD Dextran particles (red) was significantly affected by Nup160 gene silencing, indicating functional nephrocyte defects linked to abnormal NPC localization. Silencing of the Drosophila Lamin gene by nephrocyte-specific expression of a UAS-Lam-RNAi transgene driven by Dot-Gal4 (Lam-IR) led to marked abnormalities in nuclear size and shape and a severe functional deficit, but NPC localization appeared normal. (B) Mab ADL67.10 labels all isoforms of Drosophila Sf9 Lamin but does not label Lamin C. In control nephrocytes, Lamin (green) appeared as a sharp regular ring around the nucleus. Nup160 gene silencing (Nup160-IR), by contrast, did not disrupt circumferential nuclear Lamin localization (although nuclear morphology was altered as indicated by DAPI staining), but labeling was less concise and uniform than in control nephrocytes.