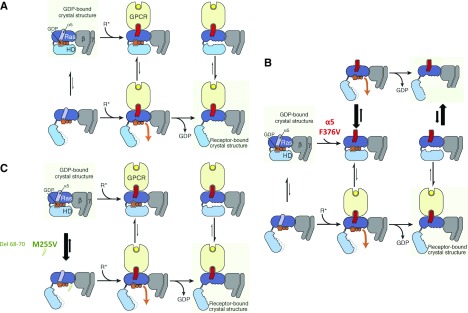
Figure 2.
Schematic view of the G protein activation process for the wild type and GNAS variants. (A) Proposed mechanism of receptor-catalyzed nucleotide release (from ref. 7). (Left panels) The rat sarcoma (Ras) and helical domains separate frequently, even in the absence of a receptor, but such separation does not usually lead to guanosine diphosphate (GDP) release. (Upper left panel) This rapid (relative to overall GDP release) equilibrium favors the closed conformation. (Center panels) Binding of an activated receptor (R*) favors a Ras domain conformational change—displacement of α5 away from GDP—that weakens interactions between GDP and the Ras domain, allowing (lower center panel) GDP to escape when the Gα-domains happen to spontaneously separate. (Right panels) Loss of GDP shifts the equilibrium toward Gα-conformations with widely separated domains (lower right panel). Reprinted from ref. 7, with permission. (B and C) Proposed mechanism of gain of function with Gαs mutation F376V, M255V, or del 68–70 (using the same format as in A). (B) The F376V α5 mutation leads to the destabilization of the C-terminal helix (middle panels; red), thus mimicking the binding of an activated receptor (i.e., favoring a Ras domain conformational change—displacement of α5 away from GDP—that weakens interactions between GDP and the Ras domain, allowing GDP to escape when the Gα-domains happen to spontaneously separate) (top panels). An activated receptor can also bind to the mutated Gs protein (bottom panels). (Right panels) Loss of GDP shifts the equilibrium toward Gα-conformations with widely separated domains in the absence (right top panel) or the presence of an activated receptor (right bottom panel). The absence of effects of tolvaptan could be explained by the presence of Gs activated independently from the vasopressin V2 receptor (right top panel). (C) The M255V (or del 68–70) mutations favor the open conformation (unlike the wild-type Gs), thus shifting the equilibrium toward Gα-conformations with widely separated domains (lower panels), and thus, favor the receptor–Gα-protein complex with widely separated domains, resulting in higher G-protein activity on loss of GDP (right panels). GPCR, G protein–coupled receptor.