Abstract
Ethnicity and culture represent important factors in shaping psychopathology as well as pharmacotherapeutic responses in psychiatric patients. A large body of literature, accumulated over the past several decades, demonstrates that these factors not only determine the metabolism and disposition of medications (pharmacokinetics), but also their interactions with therapeutic targets (pharmacodynamics). This article focuses on the impact of such variations on the diagnosis and treatment of depression and anxiety disorders between East and West. Genes controlling the expression of drug metabolizing enzymes as well as the function of the brain are highly polymorphic, and the patterns and distribution of these polymorphisms are typically divergent across ethnic groups. To the extent that these genetic patterns determine drug response, ethnic variations in these genetic dispositions will lead to differential responses in clinical settings. In addition, the expression of these genes is significantly influenced by environmental factors including diet as well as exposure to other natural products. Superimposed on these biological influences, culturally determined beliefs and behavioral patterns also profoundly influence patients’ expectations of treatment response, adherence, and interactions with clinicians. In addition to pharmacotherapeutic responses, emerging data also indicate that significant ethnic variations exist in genetic polymorphisms and neurobiologic correlates (biomarkers) that may be associated with the vulnerability to psychiatric disorders. These considerations argue for the importance of examining biological variations across ethnic groups, especially in the clinical context, in terms of the assessment and treatment of psychiatric patients, and in our understanding of psychiatric phenomenology and nosology.
Keywords: Biological marker, Culture, Ethnicity, Pharmacogenetics, Pharmacokinetics, Pharmacodynamics, Taiwan, Variation
Introduction
Representing salient dimensions responsible for shaping human behavior and experiences, sociocultural factors have long been recognized to exert major pathogenetic as well as pathoplastic effects on psychiatric disorders, and to significantly influence the course, outcome and treatment response of psychiatric patients. However, until recently, clinicians and researchers have tended to focus exclusively on psychosocial processes and mechanisms when investigating variations across ethnic groups, in both their psychopathological manifestations and treatment response. Inherent in this tendency is an implicit assumption that biological substrates of psychiatric phenomena are (or should be) universal, and thus their role in determining ethnic variations is minimal and perhaps negligible. Contrary to this traditional view regarding the relationship between culture and biology as being basically dichotomous in nature, much evidence indicates that they are intertwined and interactive, and that neither should be considered in vacuum. In addition, recent advances in molecular medicine show that variations (polymorphisms) in genes, including those centrally involved in the functioning of the brain, are typically unevenly distributed across ethnic groups. Emerging data indicate that such genotypic ethnic variations may also determine an individuals’ therapeutic responses, as well as vulnerability to psychiatric disorders. Together, these considerations argue for the importance of examining biological variations across ethnic groups, especially in the clinical context, in terms of the assessment and treatment of psychiatric patients [1].
This review will start with a brief survey of the literature on ethnic variations in psychopharmacologic response, including pharmacokinetic, pharmacodynamic, and pharmacokinetic aspects (as well as mechanisms responsible for such variations). This is followed by sections focusing on ethnic variations in psychiatric diseases and neurobiologic correlates (biomarkers) that may be relevant to psychiatric phenomenology and nosology. Findings in the former area (psychopharmacologic response) tend to be more definitive, with many of them possibly ready for clinical application (i.e., pharmacogenomics and individualized medicine). In contrast, researches in the latter (e.g., psychiatric diseases and biomarkers) are recently emerging and tend to be tentative but intriguing nonetheless. Since the relevant fields are rapidly evolving, many new findings will be available by the time this article is published. Thus, this review is not meant to be comprehensive or definitive; rather it represents a general survey of major issues and directions in the area.
Psychopharmacological Response
Substantial and often dramatic racial and ethnic variations in response to various nonpsychotropic therapies, sometimes with dire clinical consequences, have been known for close to a century [2]. For psychotropic medications, however, data confirming clinical impressions elucidating the underlying mechanisms have emerged more gradually, converging over the last decade to clearly demonstrate the existence of ethnic differences in terms of response to psychotropic therapies [3, 4]. Early reports focused on dosage and adverse event profiles and indicated that Asian patients tend to experience adverse events at lower dosages of psychotropic medications than their Caucasian counterparts [4, 5, 6]. In one study, Asians showed a greater therapeutic response than Caucasians while receiving a significantly lower mean dose of clozapine and lower mean clozapine concentrations than Caucasians; moreover, Asians were also significantly more likely to experience anticholinergic and other side effects [7]. Corresponding to these, a survey of the unit dose of medications marketed in non‐Western countries for most drugs showed that they were typically one‐quarter to one‐third of those available in Western countries, suggesting that those residing in the former countries tended to be treated with medications in dose ranges that are much lower than those in the United States and Europe [8].
Similar to what has been found in Asian groups, Hispanics might respond to tricyclic antidepressants (TCAs) with lower dosages while concurrently suffering from greater side effects [9, 10]. In addition, Escobar and Tuason [9] showed that Colombian depressed patients responded significantly better than their Caucasian counterparts to trazodone treatment. This was replicated in a collaborative study on the treatment of depression sponsored by the World Health Organization [11], indicating better response with lower doses at some sites (i.e., Japan and India), as compared to the U.S. site. Versiani et al. [12] studied depressed patients recruited from five Latin American countries (Brazil, Mexico, Peru, Colombia, and Venezuela), and found a remarkably high rate of overall response to both amitriptyline and paroxetine (74%), with amitriptyline having an additional advantage in the improvement of sleep symptoms. At the same time, patients treated with amitriptyline were also more likely to experience sedation, tremors, weight gain, and anticholinergic side effects.
Also similarly, African‐American depressed patients may be more susceptible to the effects of TCAs than their Caucasian counterparts displaying faster and more favorable clinical responses [13, 14, 15], as well as a significantly higher propensity to develop serious side effects, such as delirium [16].
Less information has been available in the literature on selective serotonin reuptake inhibitors (SSRIs). In a recent study, Chinese depressed patients were found to require lower dosages with consequently lower plasma concentrations of sertraline compared to Caucasian patients to achieve clinical efficacy [17]. As indicated above, a multisite study showed that Hispanics had an overall 74% response rate with 20 mg fluoxetine [12]. Wagner et al. [18] also reported that among HIV‐positive depressed patients, African Americans were more likely to be nonresponders to fluoxetine treatment than their Caucasian counterparts; but there were no significant differences between the two groups in their treatment emergent side effect profiles. A pooled analysis study that included 104 double‐blind, placebo‐controlled clinical trials was conducted to investigate paroxetine efficacy and safety for mood and anxiety disorder among white, black, Hispanic, and Asian subjects. It revealed that Hispanic and Asian subjects had a lower response rate, while Hispanics had the lowest and Asians had the highest full response rates. Speed of response and adverse effects were similar across groups [19].
In summary, although there are contradictory results, the literature in aggregate does support the idea that ethnicity and culture represent important factors in determining psychotropic responses. Advances in recent decades have revealed that there are indeed biological bases for such variations. While not minimizing the importance of “nonbiological” factors in influencing pharmacological practices, the biological basis for ethnic variations in pharmacological responses is now clearly established with the advent of pharmacokinetics and pharmacodynamics, and most recently pharmacogenetics and pharmacogenomics. These are briefly reviewed below.
Pharmacokinetics
A prototype of this kind of studies was a cross‐ethnic study on serum haloperidol levels in normal male volunteers [1], which measured serum haloperidol concentration in Caucasians, American‐born Asians, and foreign‐born Asians over a 7‐h period following haloperidol administration (0.5 mg given intramuscularly or 1.0 mg given orally). The results were similar between the two Asian groups but significantly different between Caucasian and Asian subjects. After controlling for body surface area, Caucasians still had lower serum haloperidol concentrations than compared to both Asian groups. A number of studies with similar designs have investigated ethnic differences in the pharmacokinetic profiles of TCAs. Kishimoto and Hollister [20], for example, compared the kinetics of a single dose of nortriptyline in Japanese and Caucasian Americans. Following a single dose of 100 mg nortriptyline, the area under the curve (AUC) for the Caucasian American subjects was 730 ng/mL/h. Although the Japanese subjects received a lower dose of 50 mg as there were concerns about possible side effects, they still achieved higher blood levels (AUC of 1150 ng/mL/h) than the Caucasian subjects. Similarly, a study in the United Kingdom compared the pharmacokinetic profiles of clomipramine in South Asian immigrants (Indian and Pakistani) with that of British Caucasians [21]. Following a single dose of 25 mg or 50 mg clomipramine, the South Asian subjects (whether residing in Asia or in England) had higher blood levels than the Caucasian subjects. However, when the study was repeated with just those South Asian immigrants who had changed to a more Western diet, the previously observed difference in blood levels compared with the Caucasian subjects disappeared. These and other studies [19, 22, 23] indicate that substantial ethnic differences in the pharmacokinetics of TCAs exist; they also suggest that such differences may be caused by both genetic and environmental (including dietary practice) factors.
Similarly, a number of carefully designed studies have also demonstrated substantial ethnic variations in the kinetics of benzodiazepines between Asians and Caucasians. Lin and his associates studied plasma alprazolam concentrations in American‐born Asian, foreign‐born Asian and Caucasian healthy male volunteers, and found that both Asian groups had lower plasma clearance and higher drug concentrations after both oral and intravenous administration of the medication [1]. In a later study with similar design, Ajir et al. [24] reported that the Asian subjects had higher serum concentrations and lower clearance of adinazolam and its metabolites than their Caucasian and African American counterparts. These and other studies [25, 26, 27] provided strong support of significant ethnic differences in response to benzodiazepines.
Two earlier studies compared antidepressant responses between African American and Caucasian patients and found substantially higher steady‐state plasma TCA concentrations in the former group when treated with the same doses of amitriptyline or nortriptyline [28, 29]. These studies also gave evidence that African Americans may be less efficient in the demethylation of tricyclics. Ziegler and Briggs [28] suggested that this slower metabolic profile might be responsible for the faster and more effective antidepressant responses reported earlier by other researchers.
Despite the widespread use of SSRIs and other newer antidepressants in Asian and Western countries, there are few studies on ethnic variations in the pharmacokinetics of these agents. In an open‐label prospective six‐week study among Australian Chinese, Malaysian Chinese, and Australian Caucasians depressed patients, the mean sertraline concentration to dose ratios at the short‐term and steady state were not significantly different between the three groups. Although there was a lack of difference in the pharmacokinetic results, Chinese depressed patients appeared to require lower dosages with consequently lower plasma concentrations of sertraline compared to Caucasian patients, to achieve clinical efficacy [17]. Similarly, there also is a paucity of information regarding the pharmacokinetics of the newer “nonbenzodiazepine” anxiolytics across ethnic groups.
Pharmacodynamics
Although there are many examples discussing ethnic differences in the pharmacodynamics of nonpsychiatric medications, including propranolol [30] and opiates [31, 32], less information is available on psychotropics in this regard. However, there are suggestions in the literature that differences may exist. In one of the studies described previously [1], prolactin‐response differences in normal Caucasian and Asian male volunteers were measured in addition to serum haloperidol concentrations. Following low doses of haloperidol, the prolactin response is elevated in a dose‐dependent manner. In this study, the Asian subjects had more prominent prolactin responses than the Caucasian subjects, and such differences remained robust after adjusting for differences in serum haloperidol concentrations between the two groups. Similarly, a lower therapeutic lithium concentration appears to more appropriate for Asian patients than for Caucasians. In the United States and Europe, the therapeutic level is between 0.8 and 1.2 mg, but in Asian patients the therapeutic level is between 0.4 and 0.8 mg [5, 33, 34, 35]. The use of a lower therapeutic level appears to be consistent across a number of Asian countries. Regarding TCAs, Hu et al. [36] reported that severely depressed hospitalized Taiwanese patients responded to substantially lower combined concentrations of imipramine and desipramine (130 ng/mL) than Caucasians as reported in the literature (180–200 ng/mL). These results suggest that in addition to pharmacokinetic factors, pharmacodynamic factors (dopamine receptor‐mediated responses) also contribute significantly to ethnic differences in response to the administration of psychotropic drugs.
Pharmacogenetics
Remarkable progress in the field of molecular genetics in the past several decades has made it clear that many of the clinically observed individual and cross‐ethnic variations in pharmacological responses, both in terms of how drugs are metabolized (part of the pharmacokinetic process) and how they act on the target organ (pharmacodynamics), have specific genetic bases. At this point, the field's understanding of the genetic mechanisms responsible for ethnic and individual variations in pharmacokinetics is extensive and still expanding. In contrast, our knowledge base with regard to the genetics responsible for variations in therapeutic target responses is less advanced, although it also is rapidly evolving. In the following paragraphs, some of the most salient findings in these areas will be highlighted.
With very few exceptions, the metabolism of psychotropics involves the cytochrome P450 enzymes, which are often responsible for the rate‐limiting step of the biotransformation and elimination of these drugs. Three of the most commonly involved in psychotropic drug metabolism, CYP2D6, CYP3A4 and CYP2C19, will be discussed here. CYP2D6 is involved in the metabolism of a large majority of the psychotropic drugs (Table 1) and has unusually complicated mutation patterns. The number of functional genes varies from zero up to 13 copies. The functional risk of mutations encoding this enzyme is important. For example, nortriptyline [37] and venlafaxine [38, 39] are metabolized very slowly in patients possessing no functional CYP2D6 gene, and are thus classified as poor metabolizers (PM). In contrast, for individuals who have multiple copies of functional genes, the drug is rapidly metabolized. Ethnic differences that exist in terms of the activity of CYP2D6 are largely determined by the genetic polymorphisms that exist across different ethnic groups. Some groups rapidly metabolize psychotropic medication and need a higher dose compared with poor metabolizer groups who are very sensitive to even low doses of medication. The frequency of the phenotype of poor metabolizers differs among ethnic groups. Less than 1% of Asians, 2–5% African‐Americans, and 6–10% Caucasians are poor metabolizers of CYP2D6 [2]. Gene duplications are found in 19–29% of Arabs and Ethiopians and 1–5% in others [40, 41]. Similarly, Luo et al. found a high frequency of gene duplication in two Israeli groups: 18% in Ethiopian Jews and 13% in Sephardic Jews. In contrast, only 6% of Yemenite Jews, and 4% of Bedouin Arabs shared this mutation [42].
Table 1.
Psychotropic drugs metabolized by CYP2D6
| Antidepressants |
| Amitriptyline, clomipramine, imipramine, desipramine nortriptyline, trimipramine, N‐desmethyl‐clomipramine fluoxetine, norfluoxetine, paroxetine, venlafaxine sertraline |
| Neuroleptics |
| Chlorpromazine, thioridazine, perphenazine, haloperidol reduced haloperidol risperidone, clozapine, sertindole |
| Others |
| Codeine, opiate, propranolol, dextromethorphan, etc. |
At the other side of the spectrum, the CYP2D6*4 allele (splice site G1934A transition) [43], which causes a truncated protein and leads to a complete loss of enzyme activity, is responsible for the bimodal distribution of the CYP2D6 phenotype, with 5–9% of Caucasians classified as poor metabolizers. This allele is extremely rare in other populations. The CYP2D6*17 allele, which contains four mutations (one silent and three amino acid exchanges T107I, R296C, and S486T) [41], results in reduced CYP2D6 activity and is found in 25–40% of sub‐Saharan African blacks and is rarely seen in those not of sub‐Saharan African origin. Similarly, the CYP2D6*10 allele (C100T mutation, resulting in a Pro34 Ser amino acid substitution) [44] also reduces the CYP2D6 activity and is found in 47–70% of Asians and 5% of others. Thus, the pharmacogenetics of CYP2D6 serves as a dramatic example demonstrating the importance of genotypic profiles in determining the phenotypic expression of the enzyme as well as the pharmacokinetics of medications metabolized by this enzyme. The advances made in molecular analysis may explain in part what many have previously observed clinically, as reviewed above. That is, Asians and African Americans are often more prone to developing side effects from psychotropics than other ethnic groups. In addition to genetic variations, the expression of CYP2D6 also could be inhibited by a large variety of commonly used medications, leading to potentially serious drug–drug interaction problems [45, 46]. These inhibitors include quinidine and cimetidine, as well as many antidepressants and neuroleptics.
CYP2C19 is another of the cytochrome P450 enzymes involved in the metabolism of a number of psychotropic drugs. Major ethnic differences exist in terms of the activity of this enzyme. Across various groups, the percentage of poor metabolizers ranges from 3% to 20%. The reduction in activity of this enzyme is caused by two specific mutations. The first is CYP2C19*2 (G681A) [47], which has a mutation in exon 5 that causes an aberrant splice site. The other variant allele is CYP2C19*3 (G636A), with a point mutation in exon 4 producing a premature stop codon. CYP2C19*3 [47] is specific to Asian individuals and is not found outside of Asian populations. The percentage of poor metabolizers has been reported to be 20% in Asians, approximately 5% in Hispanics, and approximately 3% in Caucasians [47, 48]. The rate in African‐American populations is less clear, with incidences between 4% and 19% being reported [41]. Our data [49] showed a 5.4% poor metabolizers rate in African Americans, in comparison to 16.7% in East Asians, 23.9% in Southeast Asians, 5% in Caucasians, and 3.2% in Mexican Americans. As with CYP2D6, drug–drug interaction is also an important consideration in the prescription of medications metabolized by CYP2C19. Fluvoxamine is a potent inhibitor of 2C19 along with other SSRIs, such as paroxetine and fluoxetine. Medications for the management of anxiety that are metabolized by CYP2C19 include diazepam, imipramine, amitriptyline, clomipramine, and citalopram.
CYP3A4 is also involved in the metabolism of the majority of available drugs (Table 2). The role of this enzyme in drug metabolism has attracted attention from both physicians and pharmaceutical companies in recent years, as there have been incidences of fatal drug interactions related to this enzyme, which have resulted in the withdrawal of some drugs from the market. An example of ethnic differences in the activity of this enzyme comes from a study examining the metabolism of the calcium channel blocker, nifedipine [50, 51]. Asian Indians were found to metabolize nifedipine at a slower rate than British Caucasians. As described above, another study reported similar differences between Caucasian and East Asian male volunteers in the rate of metabolism of alprazolam [1]. In this study, Asian volunteers had a higher AUC (183 ng/mL/h in American‐born Asians, 173 ng/mL/h in Foreign‐born Asians) than the Caucasian subjects (138 ng/mL/h) following single dose oral administration of the same dose (0.5 mg) after adjustment for body surface area. Alprazolam is mostly metabolized by CYP3A4. It is reasonable to speculate that there are ethnic differences in the activity of this enzyme.
Table 2.
Drugs metabolized by CYP3A4
| Typical Antipsychotics |
| Thioridazine, haloperidol |
| Atypical Antipsychotics |
| Clozapine, quetiapine, risperidone, sertindole, ziprasidone |
| Antidepressants |
| Nefazadone, sertraline, mirtazepine, tricyclic antidepressants |
| Mood Stabilizers |
| Carbamazepine, gabapentin, lamotrigine |
| Benzodiazepines |
| Alprazolam, clonazepam, diazepam, midazolam, triazolam, zolpidem |
| Calcium Channel Blockers |
| Diltiazem, nifedipine, nimodipine, verapamil |
Besides the genetic influences, the activity of drug metabolic enzymes is also determined by environmental factors. Branch et al. [52] reported a significantly longer antipyrine half‐life among Sudanese living in their home villages as compared to Sudanese residing in Britain and to white British subjects. Similar findings were reported in subsequent studies involving South Asians living in Asia, South Asian immigrants residing in Britain, and white British subjects [53, 54]. With clomipramine and antipyrine as test drugs, these studies found that immigrants who continued to follow their traditional vegetarian diet exhibited pharmacokinetic profiles similar to their brethren in Asia. In contrast, those who had switched to a British diet showed significantly faster rates of metabolism. These examples demonstrate that both genetic and environmental factors are involved in determining the activity of this and other cytochrome enzymes. Distinct patterns of genetic polymorphisms exist across ethnic groups; these can be tested and investigated alongside possible environmental and dietary factors that may cause differential expression of these genes.
Polymorphisms in those genes controlling the function of neurotransmitter systems (e.g., transporters, receptors, etc.) are thought to be related to the pathogenesis of many psychiatric disorders as well as to temperament, personality disorders and personality traits (Table 3). A case in point is the serotonin transporter gene promoter region polymorphism (5‐HTTLPR), which appears to be correlated with antidepressant response [55, 56, 57, 58, 59] as well as vulnerability for a wide range of psychiatric disorders including depression, anxiety, and suicidal risks [60, 61]. The association between this polymorphism and antidepressant response is particularly intriguing. Since 1998, at least five groups in Italy and North America (with predominant Caucasian patient populations) showed that the 5‐HTTLPR long (l) allele is associated with better or more rapid SSRIs response [55, 56, 57, 59]. Two recent studies also implicate the 5HTTLPR short (s) allele in SSRI‐emergent adverse effects [62, 63]. In contrast, in two of three studies involving Asian depressed patients [59, 64], the association was in the reverse direction: Both studies reported that the s allele was predictive of better SSRI response. In this context, it is particularly worth noting that the frequency of the 5‐HTTLPR l allele vary substantially across ethnic groups, ranging from below 20% in East Asians to greater than 70% in those with African ancestry [65] (Fig. 1). These findings together demonstrate that serotonin transporter does play a pivotal role in mediating the therapeutic effects of antidepressants, and at the same time show that its genotype may interact with ethnicity in a way that has not yet been clearly delineated. Thus, any finding derived from research conducted in a particular ethnic group should not be automatically assumed to be applicable in other groups, particularly those with especially divergent allele distribution patterns.
Table 3.
Genes with a possible association and increased susceptibility for psychiatric disorders
| Genes Encoding the Biosynthesis and Catabolism of Neurotransmitters |
| Tryptophan hydroxylase (TPH) |
| Tyrosine hydroxylase (TH) |
| Catechol‐O‐methyltransferase (COMT) |
| Monoamine oxidase (MAO) |
| Receptor Genes |
| For example, 5‐HT‐2A, 5‐HT‐1A, DRD2 |
| Transporter Genes |
| Serotonin transporter (5‐HTT) |
| Norepinephrine transporter (NET) |
| Dopamine transporter (DAT) |
Figure 1.
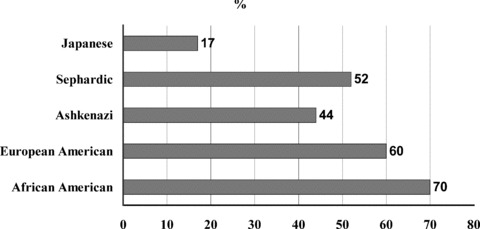
Percentage of the Serotonin transporter gene (SLC6A4) polymorphism among different populations.
Another example of significant ethnic differences in genetic variation can be found in the case of catechol‐O‐methyltransferase (COMT), which catalyses the O‐methylation of neurotransmitters, catechol hormones and drugs such as levodopa and methyldopa. Variations in COMT activity are caused by a single mutation. Thus, those homozygous for the low activity (l) allele (G1947A) [66] will have the lowest enzyme activity. Conversely, those homozygous for the high activity (h) allele will have elevated enzyme activity, with the heterozygous falling in the intermediate range. Ethnic differences in COMT activity have been observed in several populations with major differences occurring between Asian and Caucasian populations in terms of the percentage incidence of low activity COMT (18% and 50%, respectively) [67, 68]. Since COMT mediates the biotransformation of major neurotransmitters including dopamine and norepinephrine, it is not surprising that the polymorphism has been found to be associated with risks for psychopathology (including schizophrenia and mood disorders) as well as the treatment outcome of a number of neuropsychiatric disorders [69, 70, 71, 72, 73]. It is still unclear how the substantial ethnic variations in the COMT allele frequency might alter or modify such associations.
Neurobiological Correlates
As shown in the discussion above, it should be evident that ethnic variations could often be quite significant, both in terms of genetic predispositions (genotype) and clinical manifestations (clinical phenotype). What remains much less often explored is the role of ethnicity in relation to the neurobiological correlates (endophenotype) or biological markers of neuropsychiatric disorders. A number of studies, however, have dealt with markers related to depression and anxiety disorders. Some of these involved electroencephalographic (EEG) sleep patterns and also neuroendocrine changes, more specifically hypothalamo‐pituitary‐adrenal (HPA) axis activity and cortisol secretion. These will be briefly reviewed below.
Sleep Eeg Patterns
Alterations in sleep patterns and sleep disturbances are very common presenting complaints in psychiatric disorders. The total sleep time, sleep efficiency and rapid eye movement (REM) latency are all reduced in depression [74]. In contrast, REM sleep as a percentage of total sleep time is increased in depression [75]. Insomnia is also a common feature of this condition [75] and is highlighted as one of the most consistent symptoms of depression across samples in various countries. Although much is known about the changes in sleep patterns associated with psychiatric disorders, there is little information regarding the relationship between ethnicity and these changes. The data that are available suggest that there may be ethnic differences in the architecture of sleep stages. A World Health Organization (WHO) collaborative study assessed the reliability and consistency of EEG sleep abnormalities in major depression [76]. Compared with controls, depressed patients showed sleep‐continuity disturbances such as an increase in sleep‐onset latency and a decrease in total sleep time and sleep efficiency. This was in agreement with the findings of other studies [74, 75]. This study also reported that stages 2 and 3, as percentages of total sleep time, were reduced in depressed patients. Additionally, REM latency was shortened (58.44 min in depressed patients, 78.06 min in normal controls) and REM density increased. However, REM latency in depressed patients from Tokyo and Mexico City (85 and 77.4 min, respectively) was significantly different from depressed patients of other centres.
The influence of ethnicity on the manifestation of EEG sleep changes has been investigated in patients with unipolar major depression from the four ethnic groups. The sleep patterns of these patients were compared with those of age‐ and gender‐matched normal volunteers from the same ethnic groups [77]. Sleep continuity, sleep architecture and REM sleep were generally comparable among the four ethnic groups. For the normal volunteers, the results were similar to those reported by other studies. The African‐American subjects had evidence of more stage 1 and 2 sleep but had diminished stage 4 sleep whereas the Hispanic subjects had higher REM density than the Caucasian and Asian groups. Among depressed patients, the African‐American and Asian subjects had less total REM sleep and shorter REM durations during the first three REM episodes but longer REM duration during the fourth REM episode, compared with Caucasian and Hispanic subjects (Fig. 2). These findings suggest that although sleep patterns are remarkably similar across cultures, there are important cross‐ethnic differences, particularly in the depth of sleep and in phasic REM measures. However, the pathological implications of these differences remain to be explored in careful prospective studies of depressed patients from different ethnic backgrounds and in well characterized, racially matched control comparisons.
Figure 2.
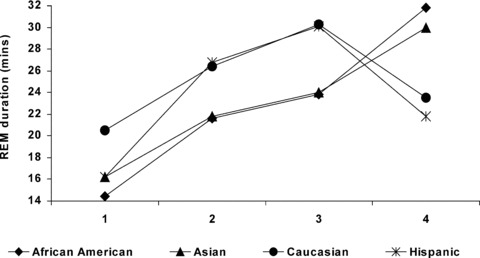
Night‐time rapid eye movement duration profiles in African‐American, Asian, Caucasian, and Hispanic patients with major depression.
Sleep paralysis is a temporary period of involuntary immobility that can occur at sleep onset or offset and is often accompanied by visual hallucination and fear. Isolated sleep paralysis (ISP) refers to sleep paralysis, which occurs without other features of narcolepsy. ISP is highly suspected to be related to sleep onset REM [78]. Prevalence rates of ISP vary among ethnic groups, with most estimates ranging from 5% to 30%, but with one estimate reaching 62%[79, 80]. The most consistently elevated rates have been reported from African‐American [81, 82], Nigerian and Sudanese [83, 84], Cambodian refugees [85], Japanese [86], and Chinese [87]. Many studies suggest a relation between ISP and posttraumatic stress disorder [88, 89, 90], panic disorder [91, 92], and anxiety disorder but not depression disorder [93]. Notably, whereas evidences of association of ISP with panic disorder have been strong among African‐Americans, they failed to reach statistical significance with Caucasians [82, 92]. The strong association of PTSD and ISP is also found in Cambodian refugees [90]. It has been suggested that there is a possibility of interaction of ISP and anxiety disorders with ethnicity [94, 95]. As in previous described, the contribution of cross‐ethnic sleep architecture differences to differential disease vulnerability, particularly in the depth of sleep and in phasic REM measures, deserve further research efforts.
HPA Axis Activity
Activation of the hypothalamic‐pituitary‐adrenal (HPA) axis is an important component of the normal stress response. Prolonged HPA overactivity occurs at all levels of this axis in depressed patients. A commonly used marker for the HPA axis function is the dexamethasone suppression test (DST), which measures suppression of cortisol; a nonsuppression rate of approximately 50% has been reported for depressed patients [96]. DST results from several studies suggest that depressed non‐Caucasian patients have different patterns of HPA axis activity compared to depressed Caucasian patients. One such study showed that depressed African‐American patients had a relatively low rate of DST nonsuppression compared with depressed Caucasian patients (25% and 58% nonsuppression, respectively) [97]. Of the eight Hispanic depressed patients included in this study, none were found to have nonsuppression. Comparable results were reported by another group who observed DST nonsuppression in 43% of depressed Caucasian patients but none of the depressed African‐American patients [98]. Other studies had similar findings and consistently suggest that DST nonsuppression is lower in depressed non‐Caucasian patients [3, 99, 100].
Our group has examined the HPA function of depressed patients and normal volunteers from four ethnic groups. Two groups of patients with chronic fatigue were also recruited. These included Caucasian and Chinese subjects. Selection criteria for the Caucasian subjects were based on the Center for Disease Control (CDC) criteria for chronic fatigue syndrome while those for the Chinese subjects were based on the ICD‐10 criteria for neurasthenia. Overall, depressed patients tended to show higher HPA activity than normal volunteers, while the chronic fatigue patients tended to show lower HPA activities compared to the normal volunteers. The results of a comparison of the free cortisol and urinary cortisol levels in these same groups suggest that there may be ethnic differences although the results did not reach a significant level. The Chinese and Caucasian patients with prominent fatigue showed lower postdexamethasone cortisol levels.
Overall, there appear to be similarities in the patterns of EEG sleep and neuroendocrine changes in depressed patients. Between the ethnic groups, statistically significant differences are consistently seen, although the interpretation of why they exist is unclear. One possible explanation is that the biological markers proposed for one group may not necessarily work well in other groups. For example, in the DST, dexamethasone is known to be metabolized at different rates in different patients; this may also be the case across ethnic groups, with the result that ethnic variations in metabolic rates affect the results of these studies. In addition, relatively few studies examining biological markers have included non‐Caucasian subjects and these have involved a small sample size. This is in contrast to the vast number of studies that have been conducted in the Western population. In order for a fair comparison to be made, additional studies with non‐Caucasian groups are needed. The results of these will provide a clearer picture of which aspect of the chronobiologic and neuroendocrine changes in depression may vary between ethnic groups.
Discussion
While it is important to investigate biological differences between populations, caution must be exercised to ensure that ethnic stereotyping does not occur. Although there are wide intergroup variations, there is also even greater intragroup variations within each ethnic group. It is important for the psychiatrist to be mindful of such variations at both individual and cross‐ethnic levels, and treat his/her patient as an individual. For example, in the haloperidol study [1], most of the Caucasian subjects had lower blood levels of haloperidol but within the groups there was variation with some overlap. Some of the Caucasian subjects had higher blood levels, while some Asian individuals had low blood levels. So it cannot be generalized that Asian patients always need a lower dose of haloperidol than Caucasian patients. However, patients from different ethnic and/or environmental backgrounds may respond differently to psychotropic drugs. The underlying psychogenic or biochemical causes of mental illness may vary between different cultures and create differences in the presenting symptomatology. The psychotropic effect of drugs may then differ or be interpreted differently. Also, the pharmacokinetics of drugs, particularly rates of metabolism, may differ due to genetic and/or environmental factors. The relevance of such differences to general clinical treatment will depend on the type of drug. If the margin of safety of a particular compound is small, then variation due to ethnic origin may be an important factor. Clinical findings should not therefore be extrapolated from one culture to another without examination of relevant ethnic groups.
Although striking ethnic differences in pharmacological responses to various medications have been documented in the general medical literature, there is a paucity of such information in the psychopharmacological literature. Recent work has provided a number of studies that illustrate interesting interethnic pharmacogenetic, pharmacokinetic, and pharmacodynamic differences. Future studies should explore newer assay methods and imaging techniques capable of measuring receptor‐drug interactions. They should also utilize existing research methodologies to more systematically scrutinize the nature and extent of such differences. They should be designed not only to ascertain differences in drug responses, but also to examine genetic and environmental (e.g., diet, exposure to enzyme inducers) factors that may contribute to these differences. Pharmacogenetic probes could be used in combination with studies examining pharmacokinetic and pharmacodynamic issues for such purposes.
Taken together, the literature clearly indicates that the disposition and effect of a large number of psychotropic agents are influenced substantially by ethnicity and culture. Recent advances in the realm of pharmacokinetics, pharmacogenetics, and pharmacodynamics have led to a greater understanding of some of the mechanisms responsible for such differences. Racial and ethnic differences in response to psychotropic medication, such as higher blood levels among some ethnic populations, affect dosage requirements and potential side effects. These findings highlight the importance of considering ethnic and racial factors in psychiatric research.
Conflict of Interest
The authors declare no conflict of interest.
References
- 1. Lin KM, Lau JK, Smith R, Phillips P, Antal E, Poland RE. Comparison of alprazolam plasma levels in normal Asian and Caucasian male volunteers. Psychopharmacology (Berl) 1988;96:365–369. [DOI] [PubMed] [Google Scholar]
- 2. Kalow W, Otton SV, Kadar D, Endrenyi L, Inaba T. Ethnic difference in drug metabolism: Debrisoquine 4‐hydroxylation in Caucasians and Orientals. Can J Physiol Pharmacol 1980;58:1142–1144. [DOI] [PubMed] [Google Scholar]
- 3. Lawson WB. Racial and ethnic factors in psychiatric research. Hosp Community Psychiatry 1986;37:50–54. [DOI] [PubMed] [Google Scholar]
- 4. Lin KM. Biological differences in depression and anxiety across races and ethnic groups. J Clin Psychiatry 2001;62(Suppl 13):13–19; discussion 20–21. [PubMed] [Google Scholar]
- 5. Takahashi R. Lithium treatment in affective disorders: Therapeutic plasma level. Psychopharmacol Bull 1979;15:32–35. [PubMed] [Google Scholar]
- 6. Yamashita I, Asano Y. Tricyclic antidepressants: Therapeutic plasma level. Psychopharmacol Bull 1979;15:40–41. [PubMed] [Google Scholar]
- 7. Matsuda KT, Cho MC, Lin KM, Smith MW, Young AS, Adams JA. Clozapine dosage, serum levels, efficacy, and side‐effect profiles: A comparison of Korean‐American and Caucasian patients. Psychopharmacol Bull 1996;32:253–257. [PubMed] [Google Scholar]
- 8. Darmansjah I, Muchtar A. Dose–response variation among different populations. Clin Pharmacol Ther 1992;52:449–452. [DOI] [PubMed] [Google Scholar]
- 9. Escobar JI, Tuason VB. Antidepressant agents—A cross‐cultural study. Psychopharmacol Bull 1980;16:49–52. [PubMed] [Google Scholar]
- 10. Marcos LR, Cancro R. Pharmacotherapy of Hispanic depressed patients: Clinical observations. Am J Psychother 1982;36:505–512. [DOI] [PubMed] [Google Scholar]
- 11. World Health Organization . Dose effects of antidepressant medication in different populations. A World Health Organization Collaborative Study. J Affect Disord 1986;2(Suppl):S1–S67. [DOI] [PubMed] [Google Scholar]
- 12. Versiani M OA, Mazzotti G, Ospina J, Davila J, Mata S, Pacheco A, Plewes J, Tamura R, Palacios M. A double‐blind comparison of fluoxetine and amitriptyline in the treatment of major depression with associated anxiety (‘anxious depression’). Cross Cultural Psychiatry 1999:249–258. [DOI] [PubMed] [Google Scholar]
- 13. Overall JE, Hollister LE, Kimbell I Jr., Shelton J. Extrinsic factors influencing responses to psychotherapeutic drugs. Arch Gen Psychiatry 1969;21:89–94. [DOI] [PubMed] [Google Scholar]
- 14. Henry BW, Overall JE, Markette JR. Comparison of major drug therapies for alleviation of anxiety and depression. Dis Nerv Syst 1971;32:655–667. [PubMed] [Google Scholar]
- 15. Raskin A, Crook TH. Antidepressants in black and white inpatients. Differential response to a controlled trial of chlorpromazine and imipramine. Arch Gen Psychiatry 1975;32:643–649. [DOI] [PubMed] [Google Scholar]
- 16. Livingston RL, Zucker DK, Isenberg K, Wetzel RD. Tricyclic antidepressants and delirium. J Clin Psychiatry 1983;44:173–176. [PubMed] [Google Scholar]
- 17. Hong Ng C, Norman TR, Naing KO, et al A comparative study of sertraline dosages, plasma concentrations, efficacy and adverse reactions in Chinese versus Caucasian patients. Int Clin Psychopharmacol 2006;21:87–92. [DOI] [PubMed] [Google Scholar]
- 18. Wagner GJ, Maguen S, Rabkin JG. Ethnic differences in response to fluoxetine in a controlled trial with depressed HIV‐positive patients. Psychiatr Serv 1998;49:239–240. [DOI] [PubMed] [Google Scholar]
- 19. Roy‐Byrne PP, Perera P, Pitts CD, Christi JA. Paroxetine response and tolerability among ethnic minority patients with mood or anxiety disorders: A pooled analysis. J Clin Psychiatry 2005;66:1228–1233. [DOI] [PubMed] [Google Scholar]
- 20. Kishimoto A, Hollister LE. Nortriptyline kinetics in Japanese and Americans. J Clin Psychopharmacol 1984;4:171–172. [DOI] [PubMed] [Google Scholar]
- 21. Lewis P, Rack PH, Vaddadi KS, Allen JJ. Ethnic differences in drug response. Postgrad Med J 1980;56(Suppl 1):46–49. [PubMed] [Google Scholar]
- 22. Rudorfer MV. Pharmacokinetics of psychotropic drugs in special populations. J Clin Psychiatry 1993;54(Suppl):50–54; discussion 5–6. [PubMed] [Google Scholar]
- 23. Schneider L, Pawluczyk B, Dopheide J, Lyness SA, Suckow RF, Cooper TB. Ethnic differences in nortriptyline metabolism (New Research Program and Abstracts, p 206). Paper presented at the annual meeting of the American Psychiatric Association. New Orleans , LA ; 1991.
- 24. Ajir K, Smith M, Lin KM, Fleishaker JC, Chambers JH, Anderson D, Nuccio C, Zheng YP. The pharmacokinetics and pharmacodynamics of adinazolam: Multi‐ethnic comparisons. Psychopharmacology (Berl) 1997;129:265–270. [DOI] [PubMed] [Google Scholar]
- 25. Ghoneim MM, Korttila K, Chiang CK, Jacobs L, Schoenwald RD, Mewaldt SP, Kayaba KO. Diazepam effects and kinetics in Caucasians and Orientals. Clin Pharmacol Ther 1981;29:749–756. [DOI] [PubMed] [Google Scholar]
- 26. Kumana CR, Lauder IJ, Chan M, Ko W, Lin HJ. Differences in diazepam pharmacokinetics in Chinese and white Caucasians—Relation to body lipid stores. Eur J Clin Pharmacol 1987;32:211–215. [DOI] [PubMed] [Google Scholar]
- 27. You JS, Hu SY, Chen B, Zhang HG. Serotonin transporter and tryptophan hydroxylase gene polymorphisms in Chinese patients with generalized anxiety disorder. Psychiatr Genet 2005;15:7–11. [DOI] [PubMed] [Google Scholar]
- 28. Ziegler VE, Biggs JT. Tricyclic plasma levels. Effect of age, race, sex, and smoking. Jama 1977;238:2167–2169. [DOI] [PubMed] [Google Scholar]
- 29. Rudorfer MV, Robins E. Amitriptyline overdose: Clinical effects on tricyclic antidepressant plasma levels. J Clin Psychiatry 1982;43:457–460. [PubMed] [Google Scholar]
- 30. Zhou Z, Roy A, Lipsky R, Kuchipudi K, Zhu G, Taubman J, Virkkunen M, Goldman D. Haplotype‐based linkage of tryptophan hydroxylase 2 to suicide attempt, major depression, and cerebrospinal fluid 5‐hydroxyindoleacetic acid in 4 populations. Arch Gen Psychiatry 2005;62:1109–1118. [DOI] [PubMed] [Google Scholar]
- 31. Cepeda MS, Farrar JT, Roa JH, Boston R, Meng QC, Ruiz F, Carr DB, Strom BL. Ethnicity influences morphine pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther 2001;70:351–361. [PubMed] [Google Scholar]
- 32. Zhou HH, Sheller JR, Nu H, Wood M, Wood AJ. Ethnic differences in response to morphine. Clin Pharmacol Ther 1993;54:507–513. [DOI] [PubMed] [Google Scholar]
- 33. Chang S PG, Yang Y, Yeh E, Davis JM. Lithium pharmacokinetics: Inter‐racial comparison. Presented at the 138th Annual Meeting of the American Psychiatric Association; 1985.
- 34. Lim L, Ng TP, Chua HC, Chiam PC, Won V, Lee T, Fong C, Kua EH. Generalised anxiety disorder in Singapore: Prevalence, co‐morbidity and risk factors in a multi‐ethnic population. Soc Psychiatry Psychiatr Epidemiol 2005;40:972–979. [DOI] [PubMed] [Google Scholar]
- 35. Yang YY. Prophylactic efficacy of lithium and its effective plasma levels in Chinese bipolar patients. Acta Psychiatr Scand 1985;71:171–175. [DOI] [PubMed] [Google Scholar]
- 36. Hu WH, Lee CF, Yang YY, Tseng YT. Imipramine plasma levels and clinical response. Bull Chin Social Neurosci Psychiatr 1983;9:40–49. [Google Scholar]
- 37. Dalen P, Dahl ML, Ruiz ML, Nordin J, Bertilsson L. 10‐Hydroxylation of nortriptyline in white persons with 0, 1, 2, 3, and 13 functional CYP2D6 genes. Clin Pharmacol Ther 1998;63:444–452. [DOI] [PubMed] [Google Scholar]
- 38. Fukuda T, Nishida Y, Imaoka S, Hiroi T, Naohara M, Funae Y, Azuma J. The decreased in vivo clearance of CYP2D6 substrates by CYP2D6*10 might be caused not only by the low‐expression but also by low affinity of CYP2D6. Arch Biochem Biophys 2000;380:303–308. [DOI] [PubMed] [Google Scholar]
- 39. Veefkind AH, Haffmans PM, Hoencamp E. Venlafaxine serum levels and CYP2D6 genotype. Ther Drug Monit 2000;22:202–208. [DOI] [PubMed] [Google Scholar]
- 40. Aklillu E, Persson I, Bertilsson L, Johansson I, Rodrigues F, Ingelman‐Sundberg M. Frequent distribution of ultrarapid metabolizers of debrisoquine in an ethiopian population carrying duplicated and multiduplicated functional CYP2D6 alleles. J Pharmacol Exp Ther 1996;278:441–446. [PubMed] [Google Scholar]
- 41. Masimirembwa CM, Hasler JA. Genetic polymorphism of drug metabolising enzymes in African populations: Implications for the use of neuroleptics and antidepressants. Brain Res Bull 1997;44:561–571. [DOI] [PubMed] [Google Scholar]
- 42. Luo HR, Aloumanis V, Lin KM, Gurwitz D, Wan YJ. Polymorphisms of CYP2C19 and CYP2D6 in Israeli ethnic groups. Am J Pharmacogenomics 2004;4:395–401. [DOI] [PubMed] [Google Scholar]
- 43. Gough AC, Miles JS, Spurr NK, Moss JE, Gaedigk A, Eichelbaum M, Wolf CR. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature 1990;347:773–776. [DOI] [PubMed] [Google Scholar]
- 44. Wang SL, Huang JD, Lai MD, Liu BH, Lai ML. Molecular basis of genetic variation in debrisoquin hydroxylation in Chinese subjects: Polymorphism in RFLP and DNA sequence of CYP2D6. Clin Pharmacol Ther 1993;53:410–418. [DOI] [PubMed] [Google Scholar]
- 45. DeVane CL. Pharmacogenetics and drug metabolism of newer antidepressant agents. J Clin Psychiatry 1994;55(Suppl):38–45; discussion 6–7. [PubMed] [Google Scholar]
- 46. Ereshefsky L, Dugan D. Review of the pharmacokinetics, pharmacogenetics, and drug interaction potential of antidepressants: Focus on venlafaxine. Depress Anxiety 2000;12(Suppl 1):30–44. [DOI] [PubMed] [Google Scholar]
- 47. De Morais SM, Wilkinson GR, Blaisdell J, Nakamura K, Meyer UA, Goldstein JA. The major genetic defect responsible for the polymorphism of S‐mephenytoin metabolism in humans. J Biol Chem 1994;269:15419–15422. [PubMed] [Google Scholar]
- 48. Goldstein JA, Ishizaki T, Chiba K, De Morais SM, Bell D, Krahn PM, Evans DA. Frequencies of the defective CYP2C19 alleles responsible for the mephenytoin poor metabolizer phenotype in various Oriental, Caucasian, Saudi Arabian and American black populations. Pharmacogenetics 1997;7:59–64. [DOI] [PubMed] [Google Scholar]
- 49. Luo HR, Poland RE, Lin KM, Wan YJ. Genetic polymorphism of cytochrome P450 2C19 in Mexican Americans: A cross‐ethnic comparative study. Clin Pharmacol Ther 2006;80:33–40. [DOI] [PubMed] [Google Scholar]
- 50. Rashid TJ, Martin U, Clarke H, Waller DG, Renwick AG, George CF. Factors affecting the absolute bioavailability of nifedipine. Br J Clin Pharmacol 1995;40:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sowunmi A, Rashid TJ, Akinyinka OO, Renwick AG. Ethnic differences in nifedipine kinetics: Comparisons between Nigerians, Caucasians and South Asians. Br J Clin Pharmacol 1995;40:489–493. [PMC free article] [PubMed] [Google Scholar]
- 52. Branch RA, Salih SY, Homeida M. Racial differences in drug metabolizing ability: A study with antipyrine in the Sudan. Clin Pharmacol Ther 1978;24:283–286. [DOI] [PubMed] [Google Scholar]
- 53. Allen JJ, Rack PH, Vaddadi KS. Differences in the effects of clomipramine on English and Asian volunteers. Preliminary report on a pilot study. Postgrad Med J 1977;53(Suppl 4):79–86. [PubMed] [Google Scholar]
- 54. Fraser HS, Mucklow JC, Bulpitt CJ, Kahn C, Mould G, Dollery CT. Environmental factors affecting antipyrine metabolism in London factory and office workers. Br J Clin Pharmacol 1979;7:237–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pollock BG, Ferrell RE, Mulsant BH, et al Allelic variation in the serotonin transporter promoter affects onset of paroxetine treatment response in late‐life depression. Neuropsychopharmacology 2000;23:587–590. [DOI] [PubMed] [Google Scholar]
- 56. Rausch JL, Johnson ME, Fei YJ, Li JQ, Shendarkar N, Hobby HM, Ganapathy V, Leiback FH. Initial conditions of serotonin transporter kinetics and genotype: Influence on SSRI treatment trial outcome. Biol Psychiatry 2002;51:723–732. [DOI] [PubMed] [Google Scholar]
- 57. Serretti A, Zanardi R, Rossini D, Cusin C, Lilli R, Smeraldi E. Influence of tryptophan hydroxylase and serotonin transporter genes on fluvoxamine antidepressant activity. Mol Psychiatry 2001;6:586–592. [DOI] [PubMed] [Google Scholar]
- 58. Smeraldi E, Zanardi R, Benedetti F, Di Bella D, Perez J, Catalano M. Polymorphism within the promoter of the serotonin transporter gene and antidepressant efficacy of fluvoxamine. Mol Psychiatry 1998;3:508–511. [DOI] [PubMed] [Google Scholar]
- 59. Zanardi R, Serretti A, Rossini D, Franchini L, Cusin C, Lattuada E, Dotoli D, Smeraldi E. Factors affecting fluvoxamine antidepressant activity: Influence of pindolol and 5‐HTTLPR in delusional and nondelusional depression. Biol Psychiatry 2001;50:323–330. [DOI] [PubMed] [Google Scholar]
- 60. Greenberg BD, McMahon FJ, Murphy DL. Serotonin transporter candidate gene studies in affective disorders and personality: Promises and potential pitfalls. Mol Psychiatry 1998;3:186–189. [DOI] [PubMed] [Google Scholar]
- 61. Jonsson EG, Nothen MM, Gustavsson JP, Neidt H, Bunzel R, Propping P, Sedvall GC. Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationships to monoamine metabolite concentrations in CSF of healthy volunteers. Psychiatry Res 1998;79:1–9. [DOI] [PubMed] [Google Scholar]
- 62. Mundo E, Walker M, Cate T, Macciardi F, Kennedy JL. The role of serotonin transporter protein gene in antidepressant‐induced mania in bipolar disorder: Preliminary findings. Arch Gen Psychiatry 2001;58:539–544. [DOI] [PubMed] [Google Scholar]
- 63. Perlis RH, Mischoulon D, Smoller JW, et al Serotonin transporter polymorphisms and adverse effects with fluoxetine treatment. Biological Psychiatry 2003;54:879–883. [DOI] [PubMed] [Google Scholar]
- 64. Kim K, Johnson JA, Derendorf H. Differences in drug pharmacokinetics between East Asians and Caucasians and the role of genetic polymorphisms. J Clin Pharmacol 2004;44:1083–1105. [DOI] [PubMed] [Google Scholar]
- 65. Stein MB, Schork NJ, Gelernter J. A polymorphism of the beta1‐adrenergic receptor is associated with low extraversion. Biol Psychiatry 2004;56:217–224. [DOI] [PubMed] [Google Scholar]
- 66. Grossman MH, Emanuel BS, Budarf ML. Chromosomal mapping of the human catechol‐O‐methyltransferase gene to 22q11.1–>q11.2. Genomics 1992;12:822–825. [DOI] [PubMed] [Google Scholar]
- 67. Ma MK, Woo MH, McLeod HL. Genetic basis of drug metabolism. Am J Health Syst Pharm 2002;59:2061–2069. [DOI] [PubMed] [Google Scholar]
- 68. Palmatier MA, Kang AM, Kidd KK. Global variation in the frequencies of functionally different catechol‐O‐methyltransferase alleles. Biol Psychiatry 1999;46:557–567. [DOI] [PubMed] [Google Scholar]
- 69. Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Kirmayer LJ, Lepine JP, Lin KM, Tajima O, Ono Y. Consensus statement on transcultural issues in depression and anxiety from the International Consensus Group on Depression and Anxiety. J Clin Psychiatry 2001;62(Suppl 13):47–55. [PubMed] [Google Scholar]
- 70. Henderson AS, Korten AE, Jorm AF, Jacomb PA, Christensen H, Rodgers B, Tan S, Esteal S. COMT and DRD3 polymorphisms, environmental exposures, and personality traits related to common mental disorders. Am J Med Genet 2000;96:102–107. [DOI] [PubMed] [Google Scholar]
- 71. Horowitz R, Kotler M, Shufman E, Aharoni S, Kremer I, Cohen H, Ebstein RP. Confirmation of an excess of the high enzyme activity COMT val allele in heroin addicts in a family‐based haplotype relative risk study. Am J Med Genet 2000;96:599–603. [PubMed] [Google Scholar]
- 72. Kotler M, Barak P, Cohen H, Averbuch IE, Grinshpoon A, Gritsenko I, Nemanov L, Ebstein RP. Homicidal behavior in schizophrenia associated with a genetic polymorphism determining low catechol O‐methyltransferase (COMT) activity. Am J Med Genet 1999;88:628–633. [PubMed] [Google Scholar]
- 73. Takeuchi T, Fukuda K, Sasaki Y, Inugami M, Murphy TI. Factors related to the occurrence of isolated sleep paralysis elicited during a multi‐phasic sleep‐wake schedule. Sleep 2002;25:89–96. [DOI] [PubMed] [Google Scholar]
- 74. Thase ME, Simons AD, Reynolds CF 3rd. Abnormal electroencephalographic sleep profiles in major depression: Association with response to cognitive behavior therapy. Arch Gen Psychiatry 1996;53:99–108. [DOI] [PubMed] [Google Scholar]
- 75. Kupfer DJ. Sleep research in depressive illness: Clinical implications—A tasting menu. Biol Psychiatry 1995;38:391–403. [DOI] [PubMed] [Google Scholar]
- 76. Mendlewicz J, Kerkhofs M. Sleep electroencephalography in depressive illness. A collaborative study by the World Health Organization. Br J Psychiatry 1991;159:505–509. [DOI] [PubMed] [Google Scholar]
- 77. Poland RE, Rao U, Lutchmansingh P, McCracken JT, Lesser IM, Edwards C, Lin KM. REM sleep in depression is influenced by ethnicity. Psychiatry Res 1999;88:95–105. [DOI] [PubMed] [Google Scholar]
- 78. Takeuchi T, Miyasita A, Sasaki Y, Inugami M, Fukuda K. Isolated sleep paralysis elicited by sleep interruption. Sleep 1992;15:217–225. [DOI] [PubMed] [Google Scholar]
- 79. Dahlitz M, Parkes JD. Sleep paralysis. Lancet 1993;341:406–407. [DOI] [PubMed] [Google Scholar]
- 80. Ness RC. The Old Hag phenomenon as sleep paralysis: A biocultural interpretation. Cult Med Psychiatry 1978;2:15–39. [DOI] [PubMed] [Google Scholar]
- 81. Bell CC, Shakoor B, Thompson B, Dew D, Hughley E, Mays R, Shorter‐Gooden K. Prevalence of isolated sleep paralysis in black subjects. J Natl Med Assoc 1984;76:501–508. [PMC free article] [PubMed] [Google Scholar]
- 82. Paradis CM, Friedman S, Hatch M. Isolated sleep paralysis in African Americans with panic disorder. Cult Divers Ment Health 1997;3:69–76. [PubMed] [Google Scholar]
- 83. Ohaeri JU. The prevalence of isolated sleep paralysis among a sample of Nigerian civil servants and undergraduates. Afr J Med Med Sci 1997;26:43–45. [PubMed] [Google Scholar]
- 84. Awadalla A, Al‐Fayez G, Harville M, Arikawa H, Tomeo ME, Templer DI, Underwood R. Comparative prevalence of isolated sleep paralysis in Kuwaiti, Sudanese, and American college students. Psychol Rep 2004;95:317–322. [DOI] [PubMed] [Google Scholar]
- 85. Hinton DE, Pich V, Chhean D, Pollack MH. ‘The ghost pushes you down’: Sleep paralysis‐type panic attacks in a Khmer refugee population. Transcult Psychiatry 2005;42:46–77. [DOI] [PubMed] [Google Scholar]
- 86. Fukuda K, Miyasita A, Inugami M, Ishihara K. High prevalence of isolated sleep paralysis: Kanashibari phenomenon in Japan. Sleep 1987;10:279–286. [DOI] [PubMed] [Google Scholar]
- 87. Wing YK, Lee ST, Chen CN. Sleep paralysis in Chinese: Ghost oppression phenomenon in Hong Kong. Sleep 1994;17:609–613. [DOI] [PubMed] [Google Scholar]
- 88. Ohayon MM, Shapiro CM. Sleep disturbances and psychiatric disorders associated with posttraumatic stress disorder in the general population. Compr Psychiatry 2000;41:469–478. [DOI] [PubMed] [Google Scholar]
- 89. Abrams MP, Mulligan AD, Carleton RN, Asmundson GJ. Prevalence and correlates of sleep paralysis in adults reporting childhood sexual abuse. J Anxiety Disord 2008;22:1535–1541. [DOI] [PubMed] [Google Scholar]
- 90. Hinton DE, Pich V, Chhean D, Pollack MH, McNally RJ. Sleep paralysis among Cambodian refugees: Association with PTSD diagnosis and severity. Depress Anxiety 2005;22:47–51. [DOI] [PubMed] [Google Scholar]
- 91. Bell CC, Hildreth CJ, Jenkins EJ, Carter C. The relationship of isolated sleep paralysis and panic disorder to hypertension. J Natl Med Assoc 1988;80:289–294. [PMC free article] [PubMed] [Google Scholar]
- 92. Paradis CM, Friedman S. Sleep paralysis in African Americans with panic disorder. Transcult Psychiatry 2005;42:123–134. [DOI] [PubMed] [Google Scholar]
- 93. Otto MW, Simon NM, Powers M, Hinton D, Zalta AK, Pollack MH. Rates of isolated sleep paralysis in outpatients with anxiety disorders. J Anxiety Disord 2006;20:687–693. [DOI] [PubMed] [Google Scholar]
- 94. Mellman TA, Aigbogun N, Graves RE, Lawson WB, Alim TN. Sleep paralysis and trauma, psychiatric symptoms and disorders in an adult African American population attending primary medical care. Depress Anxiety 2008;25:435–440. [DOI] [PubMed] [Google Scholar]
- 95. Ramsawh HJ, Raffa SD, White KS, Barlow DH. Risk factors for isolated sleep paralysis in an African American sample: A preliminary study. Behav Ther 2008;39:386–397. [DOI] [PubMed] [Google Scholar]
- 96. Staner L, Linkowski P, Mendlewicz J. Biological markers as classifiers for depression: A multivariate study. Prog Neuropsychopharmacol Biol Psychiatry 1994;18:899–914. [DOI] [PubMed] [Google Scholar]
- 97. Escobar JI. Are results on the dexamethasone suppression test affected by ethnic background? Am J Psychiatry 1985;142:268. [DOI] [PubMed] [Google Scholar]
- 98. Poland RE, Rubin RT, Lesser IM, Lane LA, Hart PJ. Neuroendocrine aspects of primary endogenous depression. II. Serum dexamethasone concentrations and hypothalamic‐pituitary‐adrenal cortical activity as determinants of the dexamethasone suppression test response. Arch Gen Psychiatry 1987;44:790–795. [DOI] [PubMed] [Google Scholar]
- 99. Coppen A, Harwood J, Wood K. Depression, weight loss and the dexamethasone suppression test. Br J Psychiatry 1984;145:88–90. [DOI] [PubMed] [Google Scholar]
- 100. De La Fuente JR, Sepulveda Amor J. Does ethnicity affect DST results? Am J Psychiatry 1986;143:275–276. [DOI] [PubMed] [Google Scholar]


