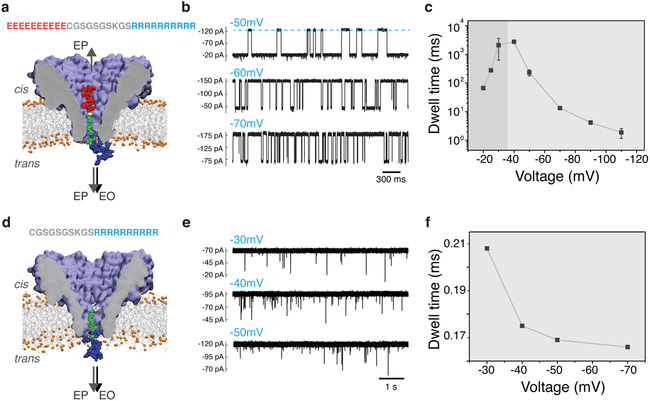
Figure 3: Experimental detection of the bipolar peptide translocation through FraC.
(a) Schematic of the peptide translocation experiment. The sequence of the bipolar peptide is listed above the molecular graphics image of the nanopore. Negative bias is applied to the trans side of the nanopore. (b) Typical current traces observed after adding 0.4 uM of bipolar peptide to the cis compartment. Transient reductions of the current indicate interactions of the individual bipolar peptides with the FraC nanopore. (c) Average duration of the current blockade (dwell time) produced by the bipolar peptide as a function of the transmembrane bias. Below approximately −40 mV, the dwell time increases with voltage indicating transient entrapment of the peptide and, likely, subsequent escape through the cis entrance of the nanopore. At voltages higher by magnitude than −40 mV, the dwell time decreases with the voltage indicating that the peptide exits the pore to the trans side. (d) Schematic representation of a control measurement performed using a truncated version of the bipolar peptide lacking the negatively charged segment (ten glutamate residues). (e) Typical current traces observed after adding 0.5 uM of the truncated peptide variant to the cis compartment. (f) Average residence time of the truncated peptide versus transmembrane bias.