Abstract
Purpose.
It is unclear whether life-expectancy estimates of patients with advanced cancer and their caregivers are associated with patient existential, social, or emotional quality of life (QOL) or caregiver emotional QOL.
Methods.
Patients with advanced cancer and their caregivers (n=162 dyads) reported estimates of the chance the patient would live for two years or more from 0% (most pessimistic) to 100% (most optimistic). They also completed self-report measures of QOL.
Results.
Adjusting for sociodemographic confounds and multiple comparisons, more pessimistic caregiver and patient life-expectancy estimates were associated with worse caregiver emotional QOL and worse patient existential QOL. Discrepancies between patient and caregiver estimates were not associated with patient or caregiver QOL.
Conclusions.
Pessimistic life-expectancy estimates are associated with worse existential QOL in patients and worse emotional QOL in caregivers. Prospective research to establish causal relationships is needed, and interventions to address the relationship between beliefs about life- expectancy and existential and emotional QOL should be considered. Providing these interventions to patients and caregivers receiving information on life-expectancy may mitigate the negative impact of life-expectancy information on patient existential quality of life.
Keywords: Cancer, oncology, caregiving, life-expectancy estimate, quality of life
BACKGROUND
The 2007 Institute of Medicine report, Caring for the whole patient: Meeting psychosocial health needs emphasized the importance of assessing and treating the psychosocial needs of patients with cancer and their informal caregivers [1]. Quality of life (QOL) is increasingly viewed as an important psychosocial construct in cancer patients and their caregivers [2] and is defined as “the effect of an illness on a patient’s physical, psychological, and social well-being as perceived by that patient (p. S560)”[3]. Cancer treatments can have a significant negative impact on patients’ QOL [4]. In the context of advanced cancer, even palliative chemotherapy has been associated with worse quality of death in patients with good performance status [5].
Cancer can also have a notable and negative impact on informal caregivers (e.g., their spouse/partner or adult child). Cancer care is increasingly provided on an outpatient basis; as a result, a greater number of caregiving responsibilities fall on informal caregivers [6]. Cancer caregivers spend an average of 32.9 hours per week on caregiving tasks and 72% perform complex medical or nursing tasks [7]. Further, many patients rely on their caregivers to manage medical appointments and information and for emotional support [8]. As a result of these tasks, over a quarter of cancer caregivers report high burden that increases as the patient’s disease progresses [9]. Given this caregiving burden, it is perhaps not surprising that cancer caregivers report poor QOL [10].
Compared to patients with early-stage disease, those with advanced cancer and their caregivers tend to experience psychological distress due to higher symptom burden and care needs, the cognitive and emotional burden of frequent and difficult treatment decisions, and, for some patients, the imminent threat of the death. The development of effective interventions to improve patient and caregiver QOL in the context of advanced cancer requires a comprehensive understanding of the factors related to QOL. Patient and caregiver understanding of illness severity is important due to its association with receipt of care consistent with patient preferences [11] and lower rates of futile aggressive care at the end of life (EOL) [12].
However, little is known about the relationship between patient and caregiver understanding of the severity of the patient’s illness and QOL. Available research examines related constructs with mixed results. Patients with advanced cancer who acknowledged the terminal nature of their illness reported worse QOL than patients who did not describe themselves as terminally ill [13, 14]. However, another study found that patients with cancer receiving palliative care who were not informed about their diagnosis also had worse QOL [15].
Even less is known about the relationship between caregiver understanding of the severity of the patient’s illness and caregiver QOL. Research on caregivers of patients with advanced cancer suggests that caregivers who are not prepared for the patient’s death are at increased risk for psychological distress in bereavement [16] and prolonged grief [17]. However, not being prepared for the death is not the same as recognizing the severity of the patient’s illness or prognosis, leaving the question of the relationship between caregiver perception of the patient’s life-expectancy and QOL unanswered.
Further, patients and caregivers experience cancer as a dyad, impacting each other in important ways [18]. In a recent study of patients with incurable cancer, the caregivers of patients who incorrectly stated that the goal of their treatment was to cure their cancer reported higher depression [19]. In addition, patients and caregivers often have different views of the patient’s illness. For example, one study found that caregivers of patients with advanced cancer were more likely than patients to report that the illness was terminal (83.4% vs. 58.0%, p<.001) [20]. A study of patients with head and neck cancer and their caregivers found that greater discrepancy in patient-caregiver perceptions of illness characteristics such as the timeline of the cancer and personal control over the cancer was associated with lower patient QOL over time [21]. These findings suggest that assessing patient and caregiver perceptions of the patient’s prognosis may provide a more comprehensive understanding of the relationship between these perceptions and patient and caregiver QOL.
The purpose of this study is to conduct secondary exploratory analyses of the relationship between individual and dyadic estimations of the patients’ life-expectancy and patient and caregiver QOL. Based on prior studies, we hypothesized that a more pessimistic patient estimation of life-expectancy would be associated with worse patient and caregiver QOL [13, 14] whereas a more pessimistic caregiver estimation would be associated with better patient and caregiver QOL [19] [21]. Further, we explored the relationships between the difference in caregivers’ and patients’ life-expectancy estimates and patient and caregiver QOL.
METHODS
Sample and procedures
Data were collected as part of a cluster randomized controlled trial examining a patientcentered communication intervention for oncologists and patients with advanced cancer and their caregivers (Values and Options In Cancer Care; VOICE) [22]. The current analyses are post-hoc secondary analyses of the data collected in the VOICE study. The present analysis is a cross-sectional examination of baseline data collected prior to initiation of the VOICE intervention; the intervention had no discernible effect on life-expectancy estimates [23]. Participants were recruited from community-based cancer clinics, academic medical centers, and community hospitals in Rochester/Buffalo, NY and Sacramento, CA. Eligible oncologists were treating patients with solid tumors and were not planning to leave the practice in the following six months. Eligibility criteria for patients included: 1) age 21 years or older, 2) diagnosis of IV non-hematological cancer or stage III cancer and whose physician “would not be surprised” if the patient were to die within 12 months [24, 25], 3) able to understand spoken English and provide written informed consent, and 4) not hospitalized or enrolled in hospice at recruitment or baseline survey administration. Eligible caregivers were: 1) non-professional or informal caregivers of enrolled patients, 2) 21 years or older, and 3) able to understand spoken English.
Oncologists were recruited at practice meetings at participating clinics. Oncologists met with study staff to complete informed consent which included agreement to assist with recruitment efforts among their patients. Patients were identified by research assistants through review of participating oncologists’ clinic rosters. Caregivers were identified by patients as a “family member, partner, friend or someone else who is involved with your health care issues, for example, someone who you talk to about personal issues including medical decisions or who comes to doctor appointments with you. This person may also help with routine day-to-day activities, like transportation or paperwork.” Study staff provided caregivers with study information and obtained informed consent. See Epstein et al., 2017 for additional information on sampling procedures [23].
Baseline measures were administered by study staff in person; patients and caregivers completed study measures separately and received $15 for each set of completed surveys. Participants were enrolled from August 2012 through June 2014 and followed through October 2015. All study methods were approved by the IRBs (IRB# RSRB00035388; clinicaltrials.gov identifier: NCT01485627) of participating sites and all participants provided written informed consent.
The present analysis is restricted to caregiver-patient dyads for which both parties provided a life-expectancy estimate, omitting dyads in which one or both members declined to answer or responded “don’t know.” Dyads in which at least one member responded “don’t know” were omitted due to the absence of data needed to interpret the meaning of this response. The sample size for the analysis of caregiver and patient estimates of patients’ lifeexpectancy was 163 (Figure 1). One caregiver with missing QOL data was excluded from the analysis of estimates of life-expectancy, reducing the final analytic sample size to N=162. Information regarding recruitment and enrollment from the larger trial has been published previously [23].
Figure 1.
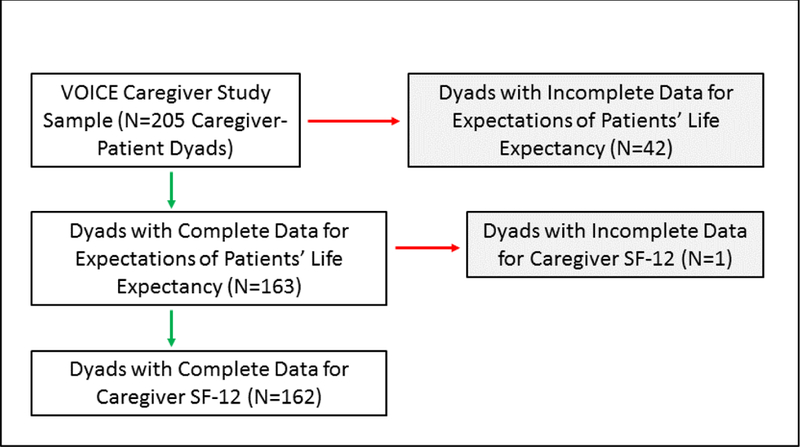
Sample sizes for assessments of estimates of patients’ life-expectancy
Measures
Study measures were chosen, in part, to minimize burden on study participants through selection of brief measures containing only items relevant to the interpretation of intervention outcomes.
Sample characteristics:
Patients self-reported age, sex, race, ethnicity, household income, education, and marital status. Cancer type was assessed by patient self-report and was dichotomized based on aggressiveness of the tumor type. “Aggressive” cancers were determined a priori in consultation with two oncologists and included lung, GI cancers (except colon), and GU cancers (except prostate). Cancers coded as less aggressive included breast, prostate, and colon cancers. Caregivers self-reported age, sex, race, ethnicity, education, marital status, and relationship to the patient.
Patient quality of life:
Patient QOL was assessed with the social subscale of the Functional Assessment of Cancer Therapy-General scale (FACT-G) [26, 27] and the emotional and existential subscales of the McGill Quality of Life Questionnaire (MQOL) [28]. These measures are widely used, have sound psychometric properties, and provide a multi-dimensional assessment of QOL. The FACT-G is a reliable and valid assessment of QOL in patients with cancer [26, 27]. Each item is rated on a five-point Likert scale (0=“not at all” to 4=“very much”); item responses are summed with higher scores indicating better QOL. The social QOL subscale consists of seven items (e.g., “I get emotional support from my family.”). The MQOL is a 16-item self-report measure of QOL over the previous two days that has been validated in individuals with life-threatening illness [29]. Participants rate each item using a 0–10 numerical response format with higher scores indicating better QOL. The emotional QOL subscale consists of four items (e.g., “Over the past two days, I have been nervous or worried.”) that are summed such that higher scores indicate better QOL. The existential QOL subscale includes six items (e.g., “Over the past two days, my life has been ‘utterly meaningless and without purpose/very purposeful and meaningful.”) that are summed such that higher scores indicate better QOL. For all QOL measures, each subscale was analyzed as a unique QOL measure.
Caregiver quality of life:
Caregiver QOL was assessed with the widely used Short Form Health Survey-12, version 2 (SF-12v2) which consists of 12-items from the SF-36 (e.g., “ How much of the time during the past 4 weeks have you felt downhearted and depressed?”) [30]. The SF-12v2 was used to calculate the emotional norm-based component summary score with higher scores indicating better QOL [30]. The SF-12v2 has adequate reliability and validity [30].
Estimations of patients’ life-expectancy:
Caregiver and patient estimations of patients’ lifeexpectancy was assessed with the question “What do you believe are the chances that (the patient) will live for 2 years or more?” [31] Response options included: “100%” (0), “about 90%” (1), “about 75%” (2), “50–50” (3), “about 25%” (4), “about 10%” (5), and “0%” (6). Higher scores (i.e., approaching 0%) were considered more pessimistic.
A two-year timeline was selected based on data from prior studies with similar recruitment methods in which advanced cancer patients had a median survival of 9–12 months [32, 33]. Recognizing the beneficial impact of novel cancer therapies on survival and the inclusion of cancers such as stage III or IV breast and prostate cancer that can be managed for extended periods, this timeline was selected to ensure that: 1) most, but not all, of the patient sample would die during the study period; 2) EOL care decisions would be paramount; and 3) differentiation between patients with pessimistic and optimistic life-expectancy estimations would be possible. The two-year timeline in this item was consistent with survival time of the patient sample; median survival in the total study sample was 16 months [23].
Caregiver-patient difference in expectations about patients’ life-expectancy was defined as the numerical difference between the caregiver’s life-expectancy estimation and the patient’s life-expectancy estimation (potentially ranging from −6 to +6) with a score of zero indicating the patient and caregiver provided the same life-expectancy estimate. Positive scores indicate that caregivers had more pessimistic expectations than patients; negative scores indicate that patients had more pessimistic expectations than caregivers.
Statistical Analysis
Frequency and descriptive (mean, standard deviation) statistics were used to describe sample characteristics and the distribution of patient and caregiver life-expectancy estimates and difference in life-expectancy estimates. Examination of the difference in caregiver and patient life-expectancy estimates was conducted using a paired sample t-test. Associations between patient and caregiver sociodemographic characteristics and life-expectancy estimates and difference in life-expectancy estimates were evaluated using Spearman (rank) correlations. Patient and caregiver sociodemographic characteristics significantly associated (p<.05) with at least one measure of life-expectancy estimates (i.e., patient, caregiver, difference) and at least one measure of QOL were considered potential confounds and controlled for in analyses of the relationship between life-expectancy estimates and QOL. Only caregiver sex met these criteria and were controlled for in subsequent analyses. Associations between caregiver and patient life-expectancy estimations and the caregiver-patient difference in expectations about patients’ life-expectancy and patient and caregiver QOL measures, adjusting for potential patient and caregiver sociodemographic confounds, were evaluated using Spearman partial correlations. A simple sequential Bonferroni-type procedure was used to account for multiple comparisons [34]. Statistical analyses were conducted using SAS statistical software, version 9.2 (Cary, NC).
RESULTS
Sample characteristics
Patients were primarily white (n=145, 89.5%), non-Latino (n=158, 97.5%), and married (n=120, 74.1%) with greater than a high school education (n=118, 72.8%). Women (n=93, 57.8%) and those 65 years of age or older (n=86, 53.1%) constituted approximately half the patient sample. Caregivers were primarily women (n=107, 66.0%), white (n=141, 87.6%), non-Latino (n=154, 95.1%), and married (n=132, 81.5%); most had attended college (n=114, 70.8%). Approximately half of the caregiver sample was 65 years of age or older (n=69, 42.6%). Two-thirds (n=104, 64.2%) of caregivers were a spouse or partner of the patient, onefifth (n=31, 19.1%) were a parent or an adult child of the patient, and the remainder (n=27, 16.7%) were friends and other relatives of the patient.
Sample characteristics and beliefs about life-expectancy
Table 1 presents patient and caregiver characteristics and their associations with lifeexpectancy expectations. Life-expectancy estimates of patients with male caregivers were more pessimistic (r=0.16, p=0.039) and more similar to their caregivers’ judgments of life-expectancy (r=−0.28, p<0.001). In addition, white caregivers reported more pessimistic patient estimates of life-expectancy (r=0.18, p=.023). A diagnosis of an aggressive cancer was associated with more pessimistic patient estimates of life-expectancy (r=0.22, p<0.006). The caregiver’s relationship to the patient was associated with caregiver-patient differences in life-expectancy estimates (r=−0.19, p=0.014) such that patients and caregivers related as spouses or partners had more similar judgments of life-expectancy than those related otherwise. No additional patient or caregiver characteristics were associated with patient, caregiver, or caregiver-patient difference in estimations of patients’ life-expectancy.
Table 1.
Patient and caregiver characteristics and their associations with caregiver, patient, and caregiver-patient difference in estimations of patient’s life-expectancy (N=162)
| Estimations of Patients’ Life-Expectancy |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Caregiver | Patient | Difference | |||||||
| N | Mean | SD | Mean | SD | Mean | SD | |||
| 162 | 1.92 | 1.79 | 1.59 | 1.67 | 0.33 | 1.98 | |||
| Patient Variable | Group | n | % | r | p | r | p | r | p |
| Age | 65+ | 86 | 53.1% | −0.02 | 0.785 | −0.12 | 0.131 | 0.09 | 0.230 |
| Less than 65 | 76 | 46.9% | |||||||
| Sex | Male | 68 | 42.2% | 0.06 | 0.480 | −0.05 | 0.548 | 0.09 | 0.256 |
| Female | 93 | 57.8% | |||||||
| Race | White | 145 | 89.5% | 0.00 | 0.982 | 0.14 | 0.079 | −0.11 | 0.164 |
| Other | 17 | 10.5% | |||||||
| Ethnicity | Latino | 4 | 2.5% | −0.03 | 0.674 | −0.13 | 0.097 | 0.08 | 0.317 |
| Non-Latino | 158 | 97.5% | |||||||
| Household Income | Up to $20,000 | 27 | 18.9% | 0.00 | 0.992 | −0.10 | 0.235 | 0.08 | 0.315 |
| Up to $50,000 | 43 | 30.1% | |||||||
| Up to $100,000 | 57 | 39.9% | |||||||
| Over $100,000 | 16 | 11.2% | |||||||
| Ed Beyond HS | Yes | 118 | 72.8% | 0.10 | 0.205 | −0.01 | 0.924 | −0.08 | 0.325 |
| No | 44 | 27.2% | |||||||
| Marital Status | Married | 120 | 74.1% | −0.03 | 0.709 | 0.01 | 0.894 | −0.10 | 0.197 |
| Not Married | 42 | 25.9% | |||||||
| Aggressive Cancer | Yes | 77 | 50.0% | 0.11 | 0.169 | 0.22 | 0.006 | −0.09 | 0.266 |
| No | 77 | 50.0% | |||||||
| Caregiver Variable | Group | n | % | r | p | r | p | r | p |
| Age | 65+ | 69 | 42.6% | −0.08 | 0.315 | −0.01 | 0.902 | −0.10 | 0.198 |
| Less than 65 | 93 | 57.4% | |||||||
| Sex | Male | 55 | 34.0% | −0.13 | 0.110 | 0.16 | 0.039 | −0.28 | 0.000 |
| Female | 107 | 66.0% | |||||||
| Race | White | 141 | 87.6% | 0.06 | 0.414 | 0.18 | 0.023 | −0.08 | 0.335 |
| Other | 20 | 12.4% | |||||||
| Ethnicity | Latino | 8 | 4.9% | 0.02 | 0.846 | −0.06 | 0.444 | 0.08 | 0.300 |
| Non-Latino | 154 | 95.1% | |||||||
| Ed Beyond HS | Yes | 114 | 70.8% | 0.14 | 0.086 | −0.03 | 0.748 | 0.14 | 0.074 |
| No | 47 | 29.2% | |||||||
| Marital Status | Married | 132 | 81.5% | 0.06 | 0.454 | 0.06 | 0.460 | 0.03 | 0.741 |
| Not Married | 30 | 18.5% | |||||||
| Relation to Patient | Spouse/Partner | 104 | 64.2% | −0.08 | 0.305 | 0.08 | 0.285 | −0.19 | 0.014 |
| NonSpouse/Part | 58 | 35.8% | |||||||
Variable coding: Age (65+=1, Less than 65=0); Sex (Male=1, Female=0); Race (White=1, Other =0); Ethnicity (Latino=1, Non-Latino=0); Household Income (Up to $20,000=1, Up to $50,000=2, Up to $100,000=3, Over $100,000=4); Ed Beyond HS (Yes=1, No=0); Marital Status (Married=1, Not Married=0); Aggressive Cancer (Yes=1, No=0); Relation to Patient (Spouse/Partner=1, Non Spouse/Partner=0).
For estimates of patients’ life-expectancy, higher scores represent more pessimistic (lower probability) estimates.
“Difference” is the numerical difference between caregiver and patient ratings of the patient’s life-expectancy (i.e., caregiver – patient).
Missing data – patient sex (1), household income (19), and aggressive cancer (8); caregiver race (1) and education (1).
Patient-caregiver difference in life-expectancy estimates
Figure 2 presents the distribution of caregiver and patient estimates of patients’ two-year life-expectancy and caregiver-patient difference on these estimates. Caregivers reported more pessimistic estimates of patients’ two-year life-expectancy than patients (mean difference=0.33, SD=1.98; t=2.14, df=161, p=0.034).
Figure 2.
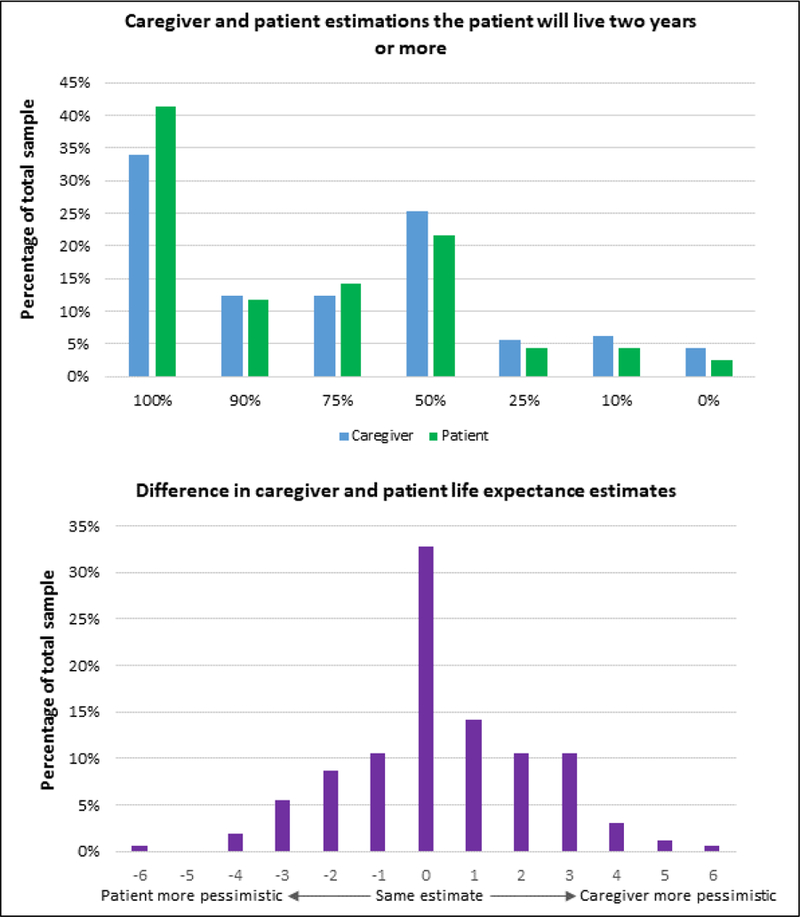
Distribution of patient and caregiver estimates of patients’ life-expectancy and difference between caregiver and patient life-expectancy estimates.
Life-expectancy estimates and patient quality of life
Table 2 presents associations between patient QOL and judgments of patients’ two-year life-expectancy, adjusting for caregiver sex. Accounting for multiple comparisons, more pessimistic caregiver estimations of life-expectancy were associated with worse existential (r=0.24, p=0.003) patient QOL but not with social (r=0.01, p=0.92) or emotional QOL (r=−0.16, p=0.039). More pessimistic patient estimations of life-expectancy were associated with worse existential (r=−0.34, p<.001) patient QOL but not with social (r=−0.17, p=0.035) or emotional (r=0.18, p=0.023) QOL. Finally, the difference in caregiver-patient life-expectancy estimates was not associated with patients’ social (r=0.17, p=0.032), emotional (r=0.00, p=0.97), or existential QOL (r=0.07, p=0.35).
Table 2.
Patient and caregiver quality of life and their associations with caregiver, patient, and caregiver-patient difference in estimations of patient’s life-expectancy (N=162)
| Estimations of Patients’ Life-Expectancy |
||||||||
|---|---|---|---|---|---|---|---|---|
| Caregiver | Patient | Difference | ||||||
| N | Mean | SD | Mean | SD | Mean | SD | ||
| 162 | 1.92 | 1.79 | 1.59 | 1.67 | 0.33 | 1.98 | ||
| Patient QoL | Mean | SD | r | p | r | p | r | p |
| Social (FACT) | 23.1 | 4.2 | 0.01 | 0.919 | −0.17 | 0.035 | 0.17 | 0.032 |
| Emotional (McGill) | 28.6 | 9.9 | −0.16 | 0.039 | −0.18 | 0.023 | 0.00 | 0.974 |
| Existential (McGill) | 44.3 | 11.3 | −0.24a | 0.003 | −0.34a | 0.000 | 0.07 | 0.346 |
| Caregiver QoL | Mean | SD | r | p | r | p | r | p |
| Emotional (SF-12) | 49.4 | 11.4 | −0.24a | 0.002 | −0.22a | 0.005 | −0.03 | 0.752 |
For estimates of patients’ life-expectancy, higher scores represent more pessimistic (lower probability) estimates. For quality of life, higher scores represent better quality of life.
“Difference” is the numerical difference between caregiver and patient ratings of the patient’s life-expectancy (i.e., caregiver – patient). A positive difference score indicates that the caregiver reported a more pessimistic life-expectancy estimate than the patient. A negative difference score indicates that the patient reported a more pessimistic estimate than the caregiver.
Correlations adjusted for caregiver sex.
Statistically significant accounting for multiple comparisons.
Life-expectancy estimates and caregiver quality of life
Table 2 also includes associations between caregiver QOL and judgments of patients’ two-year life-expectancy, adjusting for caregiver sex. More pessimistic caregiver estimations of patients’ life-expectancy were associated with worse caregiver emotional QOL (r=−0.24, p=0.002). More pessimistic patient estimations of life-expectancy were also associated with worse caregiver emotional QOL (r=−0.22, p=0.005). Greater caregiver-patient difference in lifeexpectancy estimates was not significantly associated with caregiver emotional QOL (r=−0.03, p=0.75).
DISCUSSION
Study results indicate that caregivers in this sample tend to be more pessimistic than patients regarding the patient’s life-expectancy. Further, pessimistic patient and caregiver estimations of the patient’s life-expectancy were associated with worse patient existential QOL and worse caregiver emotional QOL. Greater discordance between caregiver and patient estimations was not associated with caregiver or patient QOL.
Existential QOL was the only component of patients’ quality of life associated with lifeexpectancy estimates. Existential QOL is defined as “the perception of purpose, meaning in life, and the capacity for personal growth and transcendence” (p. 208) [29]. Prior research indicates that patients with terminal illnesses can experience a loss of meaning and sense of purpose [35] and endorse a sense of demoralization [36]. Patients who perceive their life-expectancy to be limited likely have a clear view of the terminal nature of their illness; this awareness may lead them to reflect on and question the meaning and purpose of their lives.
More pessimistic caregiver life-expectancy estimates were also associated with worse patient existential quality of life. Research on the relationship between caregiver’s understanding of the patient’s illness and patient quality of life is limited. However, prior research on the interdependence of cancer patients and caregivers [18, 19] suggests that it is plausible that caregivers’ views of the patient’s illness impact the patient’s experience. Caregivers’ perception of the patient’s life-expectancy may influence their interactions with the patient in a way that prompts the patient to contemplate the meaning and purpose of their lives.
Patient and caregiver life-expectancy estimates were not associated with patient social and emotional QOL after controlling for multiple comparisons. Social QOL is grounded in a patient’s relationships with others. Patients’ and caregivers’ understanding of life-expectancy may be unknown to others, thereby having little or no discernible impact on relationship quality. Even within the patient-caregiver relationship, dyad members may be unaware of the other members’ life-expectancy estimate [37]. The non-significant relationship between lifeexpectancy estimates and patient emotional QOL is inconsistent with some previous research [13, 14]. For example, one study of patients with incurable cancer found that patients who described their illness as terminal had worse quality of life and higher levels of depression [14]. However, unlike our sample, that sample was restricted to patients with newly diagnosed lung or non-colorectal gastrointestinal cancers; differences between the study samples and assessments of emotional QOL make direct comparisons difficult.
For caregiver QOL, a more pessimistic caregiver estimate of life-expectancy was associated with worse emotional QOL. Believing that a loved one’s life-expectancy is limited is difficult and may have a negative impact on caregivers’ emotional well-being. Alternatively, caregivers with worse emotional QOL may have a more negative view of the severity of the patient’s illness, consistent with the “depressive realism hypothesis” [38] which states that nondepressed individuals are biased toward a positive outcome. Caregivers with worse emotional QOL may be less influenced by the bias, resulting in more pessimistic life-expectancy estimates.
Research on the relationship between patients’ perceptions of their illness and caregiver well-being is limited and findings are mixed. In the current study, more pessimistic patient estimations of life-expectancy were associated with worse caregiver emotional QOL. In contrast, a recent examination of patients with advanced thoracic and gastrointestinal cancers found that overly optimistic patient perceptions of prognosis were associated with greater caregiver depression [19]. Differences in study samples, operationalization of prognostic understanding (i.e., treatment intent versus 2-year life-expectancy), and constructs assessed (i.e., depression versus QOL) may explain the apparently discrepant findings.
Clinical Implications
While many factors impact patient and caregiver QOL, life-expectancy estimates are particularly relevant and unique to patients with advanced cancer and their caregivers. Given this and the relationship between illness understanding and end-of-life care received [12], assessing patient and caregiver estimates of life-expectancy and providing psychosocial support may improve QOL while also having a positive impact on care received. Research that examines the relationship between patient and caregiver estimation of prognosis and multiple domains of patient and caregiver QOL over time is needed to elucidate these findings.
More pessimistic estimations of the patient’s life-expectancy were associated with worse patient existential QOL and caregiver emotional QOL. Identifying patients and caregivers with pessimistic life-expectancy estimates and providing them with psychosocial services that provide support and strategies for coping with prognostic information may reduce the negative impact of this information on their QOL. Interventions such as meaning-centered psychotherapy [39] and dignity therapy [40] have been shown to improve spiritual well-being and existential quality of life in cancer patients. Providing these interventions to patient and caregivers receiving information on life-expectancy may mitigate the negative impact of life-expectancy information on patient existential quality of life.
Study Limitations and Future Directions
Study findings should be interpreted in the context of study limitations. First, these analyses are cross-sectional, precluding conclusions about the causal relationship between patient-caregiver estimates of life-expectancy and QOL. Further, due to the cross-sectional design and focus on patients with advanced cancer, we are unable to examine changes in lifeexpectancy estimates and quality of life over the disease trajectory. Research examining changes in the association between life expectancy estimates and quality of life across the illness trajectory, particularly at the end-of-life may provide a better understanding of how life expectancy estimates and quality of life are intertwined, with implications for the development of interventions. Second, the sample was primarily white and married with education beyond high school; the sample is not representative of the U.S. population and results cannot be generalized to other populations. For example, cultural differences across racial and ethnic groups may be related to patients’ and caregivers’ understanding of life expectancy information and/or their willingness to discuss life expectancy. Research on these differences will inform the development of culturally-sensitive interventions targeting patient and caregiver illness understanding. Further, demographic information on ineligible dyads is not available precluding examination of demographic differences between eligible and ineligible dyads. Third, given the exploratory nature of these analyses, we opted for an empirical approach to the identification of potential confounds and for a simple and straightforward analysis, partial correlations.
Fourth, dyads in which one or both members responded “don’t know” to the item assessing estimations of the patient’s life-expectancy were excluded from analyses. Information on why patients and/or caregivers responded “don’t know” was unavailable. Future research should examine why patients and caregivers report not knowing patients’ lifeexpectancy and the implications of not knowing for patient and caregiver QOL. Fifth, patients and caregivers completed different measures of QOL that did not assess identical dimensions of QOL. As a result, we were unable to examine the relationship between life-expectancy estimates and caregiver social and existential quality of life. Finally, information on clinical and treatment-related variables for the patient sample was limited to cancer type. Variables such as treatment received and time since diagnosis may influence the relationship between lifeexpectancy estimates and QOL in patients and caregivers.
In conclusion, this study adds to our knowledge of factors impacting patient and caregiver well-being in advanced cancer care. Prospective research to establish causal relationships is needed, and interventions to address the relationship between beliefs about life expectancy and existential and emotional QOL should be considered. Our findings highlight the importance of including patients and caregivers in conversations about prognosis and providing them with psychosocial services that address the impact of this information on QOL.
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to report
REFERENCES
- 1.Institute of Medicine of the National Academies (2008) Cancer care for the whole patient: Meeting psychosocial health needs Washington, DC: The National Academies Press. [PubMed] [Google Scholar]
- 2.Lechelt LA, et al. (2018) Top 10 research priorities in head and neck cancer: Results of an Alberta priority setting partnership of patients, caregivers, family members, and clinicians. Head Neck 40: 544–554. [DOI] [PubMed] [Google Scholar]
- 3.Soni MK and Cella D. (2002) Quality of life and symptom measures in oncology: An overview. The American journal of managed care 8: S560–73. [PubMed] [Google Scholar]
- 4.de Vries YC, et al. (2018) Taste and smell perception and quality of life during and after systemic therapy for breast cancer. Breast Cancer Res Treat [DOI] [PMC free article] [PubMed]
- 5.Prigerson HG, et al. (2015) Chemotherapy Use, Performance Status, and Quality of Life at the End of Life. JAMA Oncol 1: 778–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Ryn M, et al. (2011) Objective burden, resources, and other stressors among informal cancer caregivers: a hidden quality issue? Psychooncology 20: 44–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Alliance for Caregiving (2016) Cancer caregiving in the U.S.: An intense, episodic, and challenging care experience http://www.caregiving.org/wpcontent/uploads/2016/06/CancerCaregivingReport_FINAL_June-17-2016.pdf. Accessed October 17, 2016.
- 8.Cooley ME, et al. (2017) Patient and caregiver perspectives on decision support for symptom and quality of life management during cancer treatment: Implications for eHealth. Psychooncology 26: 1105–1112. [DOI] [PubMed] [Google Scholar]
- 9.Jones SB, Whitford HS, and Bond MJ. (2015) Burden on informal caregivers of elderly cancer survivors: Risk versus resilience. J Psychosoc Oncol 33: 178–98. [DOI] [PubMed] [Google Scholar]
- 10.Cubukcu M (2017) Evaluation of quality of life in caregivers who are providing home care to cancer patients. Support Care Cancer [DOI] [PubMed]
- 11.Mack JW, et al. (2010) End-of-life discussions, goal attainment, and distress at the end of life: predictors and outcomes of receipt of care consistent with preferences. J Clin Oncol 28: 1203–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Trice ED, et al. (2009) Predictors of aggressive end-of-life care among Hispanic and white advanced cancer patients. J Clin Oncol 27: 9538. [Google Scholar]
- 13.El-Jawahri A, et al. (2014) Associations among prognostic understanding, quality of life, and mood in patients with advanced cancer. Cancer 120: 278–85. [DOI] [PubMed] [Google Scholar]
- 14.Nipp RD, et al. (2017) Coping and prognostic awareness in patients With advanced cancer. J Clin Oncol 35: 2551–2557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Victor A, et al. (2018) Benefit or harm? A study on impact of collusion on the quality of life among palliative care patients. Indian J Palliat Care 24: 61–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hauksdottir A, et al. (2010) Long-term harm of low preparedness for a wife’s death from cancer--a population-based study of widowers 4–5 years after the loss. Am J Epidemiol 172: 389–96. [DOI] [PubMed] [Google Scholar]
- 17.Tsai WI, et al. (2015) Longitudinal changes and predictors of prolonged grief for bereaved family caregivers over the first 2 years after the terminally ill cancer patient’s death. Palliat Med 30: 495–503. [DOI] [PubMed] [Google Scholar]
- 18.Epstein RM (2013) Whole mind and shared mind in clinical decision-making. Patient Educ Couns 90: 200–6. [DOI] [PubMed] [Google Scholar]
- 19.Nipp RD, et al. (2016) Factors associated with depression and anxiety symptoms in family caregivers of patients with incurable cancer. Ann Oncol 27: 1607–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yun YH, et al. (2010) Experiences and attitudes of patients with terminal cancer and their family caregivers toward the disclosure of terminal illness. J Clin Oncol 28: 1950–7. [DOI] [PubMed] [Google Scholar]
- 21.Richardson AE, Morton RP, and Broadbent EA. (2016) Changes over time in head and neck cancer patients’ and caregivers’ illness perceptions and relationships with quality of life. Psychology & Health 31: 1203–1219. [DOI] [PubMed] [Google Scholar]
- 22.Hoerger M, et al. (2013) Values and options in cancer care (VOICE): Study design and rationale for a patient-centered communication and decision-making intervention for physicians, patients with advanced cancer, and their caregivers. BMC Cancer 13: 188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Epstein RM, et al. (2017) Effect of a patient-centered communication intervention on oncologist-patient communication, quality of life, and health care utilization in advanced cancer: The VOICE randomized clinical trial. JAMA Oncol 3: 92–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moss AH, et al. (2010) Prognostic significance of the “surprise” question in cancer patients. J Palliat Med 13: 837–40. [DOI] [PubMed] [Google Scholar]
- 25.Downar J, et al. (2017) The “surprise question” for predicting death in seriously ill patients: a systematic review and meta-analysis. Canadian Medical Association Journal 189: E484–e493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cella DF, et al. (1993) The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol 11: 570–9. [DOI] [PubMed] [Google Scholar]
- 27.Luckett T, et al. (2011) Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: issues, evidence and recommendations. Ann Oncol 22: 2179–90. [DOI] [PubMed] [Google Scholar]
- 28.Cohen SR, et al. (1997) Validity of the McGill Quality of Life Questionnaire in the palliative care setting: A multi-centre Canadian study demonstrating the importance of the existential domain. Palliat Med 11: 3–20. [DOI] [PubMed] [Google Scholar]
- 29.Cohen SR, et al. (1995) The McGill Quality of Life Questionnaire: a measure of quality of life appropriate for people with advanced disease. A preliminary study of validity and acceptability. Palliat Med 9: 207–19. [DOI] [PubMed] [Google Scholar]
- 30.Ware JE, User’s Manual for the SF-12v2 Health Survey (with a Supplement Documenting SF-12 Health Survey) 2002: QualityMetric Incorporated. [Google Scholar]
- 31.Gramling R, et al. (2016) Determinants of Patient-Oncologist Prognostic Discordance in Advanced Cancer. JAMA Oncol 2: 1421–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Temel JS, et al. (2010) Early palliative care for patients with metastatic non-small-cell lung cancer. N Engl J Med 363: 733–42. [DOI] [PubMed] [Google Scholar]
- 33.Bakitas MA, et al. (2015) Early Versus Delayed Initiation of Concurrent Palliative Oncology Care: Patient Outcomes in the ENABLE III Randomized Controlled Trial. J Clin Oncol 33: 1438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y and Hochberg Y. (1995) Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 57: 289–300. [Google Scholar]
- 35.Breitbart W (2002) Spirituality and meaning in supportive care: spirituality- and meaning-centered group psychotherapy interventions in advanced cancer. Support Care Cancer 10: 272–80. [DOI] [PubMed] [Google Scholar]
- 36.Robinson S, et al. (2015) A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: A decade of research. J Pain Symptom Manage 49: 595–610. [DOI] [PubMed] [Google Scholar]
- 37.Zhang AY and Siminoff LA. (2003) Silence and cancer: Why do families and patients fail to communicate? Health Communication 15: 415–429. [DOI] [PubMed] [Google Scholar]
- 38.Alloy LB and Abramson LY. (1979) Judgment of contingency in depressed and nondepressed students: sadder but wiser? J Exp Psychol Gen 108: 441–85. [DOI] [PubMed] [Google Scholar]
- 39.Breitbart W, et al. (2015) Meaning-centered group psychotherapy: an effective intervention for improving psychological well-being in patients with advanced cancer. J Clin Oncol 33: 749–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chochinov HM, et al. (2011) Effect of dignity therapy on distress and end-of-life experience in terminally ill patients: a randomised controlled trial. Lancet Oncol 12: 753–62 [DOI] [PMC free article] [PubMed] [Google Scholar]


