Abstract
In insects, the gall-inducing life-style has evolved independently many times. Several evolutionary pathways leading to this lifestyle have been proposed. While there is compelling evidence supporting surface-feeders and stem-borers as ancestral states of insect gall-inducers, an evolutionary pathway from leaf-miners remains hypothetical. Here we explored this question by comparing the developmental processes of two micromoths, a gall-inducer Caloptilia cecidophora (Lep., Gracillariidae), and its non-gall-inducing relative C. ryukyuensis. Like other Caloptilia, the first and second instars of C. cecidophora are leaf-miners and the gall is initiated inside the leaf mine by the third instar, thus suggesting leaf-mining as an ancestral, plesiomorphic state in this case. This is the first example of an insect species switching from leaf-mining to gall-inducing during larval development. The first two leaf-mining instars of C. cecidophora exhibit an absence of growth and a reduced time duration compared to C. ryukyuensis. The shortening of the duration of leaf-mining stages is apparently compensated in C. cecidophora by a larger egg size than C. ryukyuensis, and an additional larval instar during the gall phase.
Subject terms: Evolutionary ecology, Evolutionary ecology, Evolutionary ecology, Evolutionary ecology, Entomology
Introduction
In the class Insecta, the gall-inducing habit has evolved many times independently in six orders and is especially common among Hymenoptera, Diptera and Hemiptera1. Gall induction is defined as a plant growth response to stimuli produced by an inducing organism, generated through cell growth (hyperplasia) and/or cell enlargement (hypertrophy), and resulting in a phenotype specific to the gall-inducer species2. The phenotypic specificity distinguishes gall induction from non-specific foreigner-induced plant growth responses such as callus formation3. It has been hypothesized that the gall-inducing habit evolved either from the sedentary surface feeding habit or from stem boring4. Research efforts have been concentrated on the ‘sedentary’ route, where it has been shown that sawflies5, thrips6 and aphids7 that induce galls may have evolved from sedentary surface feeders. Regarding the other scenario, origins from the stem-boring habit, tephritid flies and in some caterpillars are proposed as examples4.
In addition to those two evolutionary pathways, we propose a third whereby gall induction evolved from the leaf-mining habit. Finding transitional states having gall-inducing characters in leaf-miner groups would support this third hypothesis. One such gall-inducing character is hyperplasia, which has been reported in some leaf mines8,9. In addition to the low number of cells generally produced, the nutritive role of the newly formed tissue for the insect and the role of the insect in its formation is debated8,9. Recently, we established the nutritive role of callus present in the leaf-mines of the micromoth Borboryctis euryae (Lep., Gracillariidae) and the active role played by the insect in the induction of this phenotype3. However, given that callus cells form an undifferentiated tissue, this induced phenotype cannot be considered as a phenotype specific to the gall-inducer species. Therefore, the existence of callus in leaf mines on its own cannot be considered as evidence that leaf-mining is an ancestral state of gall induction.
Here, we explore the habits of the East Asian micromoth Caloptilia cecidophora (Lep., Gracillariidae) whose larva induces galls on Glochidion obovatum, G. rubrum and G. acuminatum (Phyllanthaceae) (Fig. 1). In the Caloptilia clade that feed on Phyllanthaceae, C. cecidophora is the only species that has been described as gall-inducer10. Caloptilia larvae typically have four successive styles of leaf-mining during larval development. Serpentine mines are created by first and early second instars, blotch mines by late second instars and three-dimensional tentiform mine by third instar. The remaining larval instars leave the mine and create a shelter by forming a leaf-roll11 (Fig. S1). In C. cecidophora, however, late instar larvae make a gall instead of a leaf-roll, and it has been suggested that early larval stages of C. cecidophora might be leaf-miners10.
Figure 1.
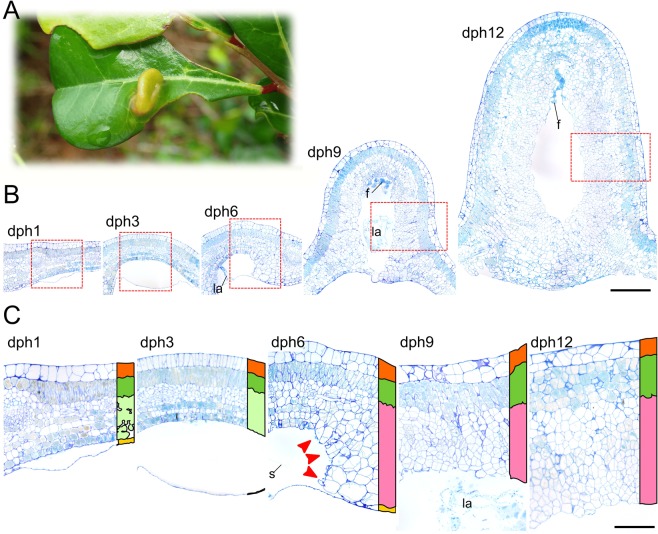
Gall initiated on Glochidion obovatum leaves attacked by Caloptilia cecidophora (A). Time series of histological sections of C. cecidophora gall initiation. (B) Cross sections of G. obovatum leaves attacked by C. cecidophora were made at 1 day post-hatching (dph1), at dph3, at dph6, at dph9, and at dph12. Red rectangles correspond to the zone detailed in (C). Legend, orange: upper epidermis, green: palisade parenchyma, light green: spongy parenchyma, yellow: lower epidermis, pink: altered parenchyma showing hypertrophied cells and an activation of cell division, s: silk, la: larvae, f: frass. Red arrows point cell division and hypertrophia orientated in direction to insect chamber. Scale: 500 µm for (B), 100 µm for (C). Staining: Toluidine Blue O.
We compared C. cecidophora larval development with that of its most closely related non-gall-inducing species, C. ryukyuensis. We propose that a gall-induction inside the mine provides support for direct evolution from leaf-mining, whereas a gall induction outside of the mine, which requires departure of the larva from the leaf mine gallery, provides support for the external feeding life-style being the ancestral state. To test these ideas, we established a time series of the histological changes that occur in mined leaves during the early stages of larval development. We aborted young larvae to test whether gall symptoms would appear in their absence. In addition, we compared the size of C. cecidophora and C. ryukyuensis at each developmental stage, in order to test the relationship between larval life-styles and patterns of larval growth.
Methods
Field collection and laboratory rearing
Caloptilia cecidophora galls on Glochidion obovatum were collected in Tomogashima Island (Wakayama, Japan; 34.28 N, 135.00 E) and maintained in a laboratory growth chamber (25 °C, 75% humidity, LP-1PH, NK system, Osaka, Japan) at Kyoto Prefectural University in Kyoto, Japan. Adults were sexed and transferred to mesh cages (40 × 40 × 40 cm) held in a greenhouse (25 °C, 70 ± 10% humidity). Each cage contained one or two pairs of moths and tissue paper soaked with a 2% sucrose solution, which was provided as a food for adults12. A branch of a potted G. obovatum bearing young leaves was placed in the cage. C. cecidophora females will only lay eggs on very fresh leaves. Oviposition was checked daily. When eggs were found, the twig was replaced. All rearing was conducted at Kyoto Prefectural University, Japan.
We also collected mines of a closely related13 non-gall-inducing species, C. ryukyuensis, on G. zeylanicum and G. lanceolatum in Ishigaki Island (Okinawa, Japan; 24.32-51 N, 124.07-27 E) and reared them on G. zeylanicum in Kyoto Prefectural University using the same protocol described above for C. cecidophora.
Histological analysis of gall ontology
We made a time series of the initial stages of the gall development by sampling attacked leaves every three days, starting when the egg hatched and continuing until the gall with its larval chamber had been formed. To analyze the internal structure of a mature gall, we sectioned field collected galls occupied by fifth instars. To test whether galls are induced by third instar larvae, we killed second instar larvae three days post hatching (dph3) and then sampled the leaf at dph6. We killed the larvae with a thin needle. Larval instars were determined by counting the number of head-capsule moults of previous instars14,15. Head capsules were collected inside the leaf mines and identified by binocular microscope and transmitted light.
Cross-sections (about 2 mm wide) were cut with a surgical scalpel and fixed for 4 h with 2.5% paraformaldehyde and 0.4% glutaraldehyde in 0.1 M McIlvaine citrate-phosphate buffer, pH 7.0. After dehydration in a graded series of ethanol, samples were embedded in medium grade LR White resin (London Resin Company Ltd, UK). Semi-thin sections (1 µm thick) were cut with a diamond knife (Diatome, Biel, Switzerland), or a triangular glass knife for the experiment in which the larva has been removed, installed in an ultracut R microtome (Leica, Rueil-Malmaison, France), placed on slides, fixed by heating at 120 °C for 2 min and stained with Toluidine Blue O 0,1% (w/v in water) (Sigma-Aldrich, T0394). Images were assembled using MosaicJ plugin of ImageJ16. Photoshop (Adobe) was used to adjust contrast and white balance, remove background, and for cropping.
Assessment of insect development
We compared the immature growth patterns of C. cecidophora with its closely related non-gall-inducing species, C. ryukyuensis, by measuring eggs (NC. cecidophora = 20 NC. ryukyuensis = 11), larvae (N comprised between 6 and 22, for details see Table S8) and pupae (NC. cecidophora = 10 NC. ryukyuensis = 7). After inducing oviposition of field-collected pairs of both species, we measured egg width and length. After hatching, we measured head-capsule width of first and second instars. Later larval instars and pupae of C. cecidophora and C. ryukyuensis were directly collected from the field (Tomogashima and Ishigaki Islands, respectively). We measured head-capsule with the micrometer of a light microscope (Leica M205C). In addition, we measured the duration of both first and second instars and filmed each stage of larval development for both species (Pentax K3, with binocular microscope Leica S6E).
The shape of the egg area was approximated being an ellipse. Area was calculated using length and width measurements. Egg areas, head-capsule widths and pupa lengths were compared using Welch’s t-tests. For the comparisons of larval head capsules among instars, we applied the sequential Bonferroni correction17 to p-values to keep the significance level at 0.05 throughout the tests. The corrected p-values are indicated as p (adjusted). All statistical analyses were conducted using RStudio version 1.1.453 (RStudio, Boston, USA) with R 3.3.1 (R Developmental Core Team 2016).
Larval head morphology before and after gall induction began, in particular mouthparts of the second and third instars of C. cecidophora, was analyzed using scanning electron microscopy. Larvae were dehydrated in ethanol overnight, mounted on scanning electron microscopy stubs, coated with gold, and observed with a scanning electron microscope (JEOL DATUM, JSM-5800LV) at an accelerating voltage of 20 kV. Images were assembled using MosaicJ plugin of ImageJ. Colors were added using Inkscape (v0.92, www.inkscape.org).
Results
Gall ontology
First and second instars of C. cecidophora mine along the lower side of the leaf (Fig. S1, Movie S2). This is also the case for C. ryukyuensis and other Caloptilia (Fig. S1, Movie S3). Analysis of the time series of cross-sections of attacked leaves (Fig. 1) revealed that the first instar makes a gallery inside the lower epidermis at one day-post hatching (dph1). This mine is enlarged into a blotch mine by the second instar, which begins dph3. The absence of any tissue alteration shows that those two instars are true leaf-miners.
The third instar begins at dph4. Two days later, at dph6, the mine induced by C. cecidophora differs from the types of mines induced by other Caloptilia species by the formation of hypertrophied cells showing periclinal divisions (i.e. cell divisions whose division plan is parallel to the leaf surface) in the spongy parenchyma located at the edges of the mine (Fig. 1). Cell hypertrophy and hyperplasia - activation of cell divisions - occurred after the insect reaches the third instar. This evidence suggests that gall induction is initiated by the third instar. Such tissue alterations are not observed in third instar mines of C. ryukyuensis. (Fig. S5).
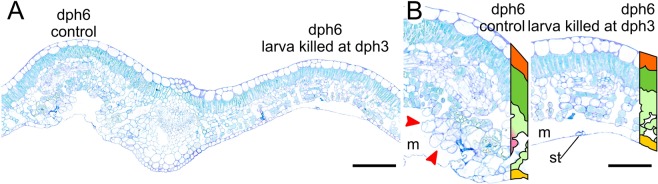
To test whether gall induction coincides with the beginning of the third instar, we killed the late second instar larva and observed the effect on the development of the leaf region attacked by the insect. Three days after killing the late second instar larva, no alteration was observed in the spongy parenchyma cells adjacent to the larva, contrary to the control leaf-mine in which the same tissue exhibits cell hyperplasia and hypertrophy (Fig. 2), demonstrating that gall induction requires the third instar larva.
Figure 2.
Histological sections of Caloptilia cecidophora gall after larva abortion. Cross sections of Glochidion obovatum leaves attacked by C. cecidophora were made at 6 day post-hatching (dph6) after killing the larva at dph3. Legend, m: mine, st: stomata, for remaining see Fig. 1. Scale: 500 µm for (A), 100 µm for (B). Staining: Toluidine Blue O.
The closure of the gall at the lower side starts with third instar using silk to fold up the leaf cuticle of the lower epidermis (Movie S2). Gall closure is completed at dph12 by the coming together of the newly formed tissues (Fig. 1). Frass accumulates in the upper region of the gallery from dph9. The presence of frass within the tissue of the same region at latter stage of gall development (Fig. 3) provides evidence that the dense parenchyma that lines the larva merges also at the upper part to form an insect chamber.
Figure 3.
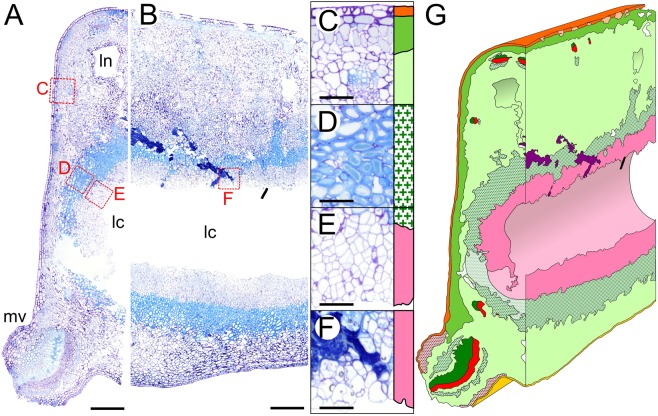
Histological sections and 3D modeling of Caloptilia cecidophora gall at late larval instar. (A) Transversal section. (B) Sagittal section. (C) Detail of gall epidermis. (D) Detail of the sclerenchyma. (E) Detail of nutritive tissue. (F) Detail of insect frass trapped in nutritive tissue. (G) 3D modeling of a mature gall based on histology sections. Legend, dark green: xylem, red: phloem, pink: nutritive tissue, green cross: scerenchyma, red cross: colenchyma, purple: frass, lc: larva chamber, ln: lacuna, mv: midvein, for remaining see Fig. 1. Scale: 500 µm for (A,B), 100 µm from (C–F). Staining: Toluidine Blue O.
In mature galls (Fig. 3), the insect chamber is lined with two kinds of tissues that are absent in the normal leaf: a sclerenchyma, and a dense parenchyma that presents traces of feeding. In addition, we found an antero-posterior asymmetry in the sagittal section of mature galls (Fig. S6). Sclerenchyma is absent in the anterior part but present in the posterior, where it co-occurs with frass. At the end of larval development and before pupation, the dense parenchyma is entirely eaten in the anterior region of the gall. A “window” of intact upper epidermis cuticle through which the adult moth escapes is appeared at the anterior surface (Fig. S7).
Larval development
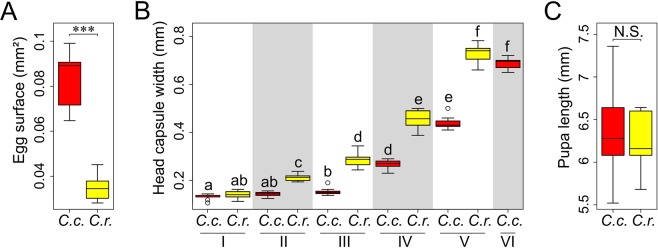
It took three days for C. cecidophora larvae to moult to the third instar (n = 8), whereas C. ryukyuensis took five days (n = 9). Eggs of C. ryukyuensis are smaller than those of C. cecidophora (t = 16.075, df = 28.542, p-value = 7.72e-16) (Fig. 4A). The first larval instars of both species have similar head capsule widths (Fig. 4B, Table S8). C. ryukyuensis larval body size increases significantly at each instar, whereas the body size of C. cecidophora larvae remains the same through the third instar (Fig. 4B). Larval size starts to increase at the fourth instar. It continues to grow during the fifth instar. C. cecidophora possess a sixth instar, an additional developmental stage in comparison with the five larval instars of C. ryukyuensis and other Caloptilia18. This extra sixth instar shows similar head capsule width as the fifth instar of C. ryukyuensis. Pupae of both species show similar body length (t = 0.37783, df = 14.996, p-value = 0.7109).
Figure 4.
Boxplots showing insect size along development of Caloptilia cecidophora (C.c., in red) and its closely related non-gall-inducing species C. ryukyuensis. (C.r., in yellow) (A) Egg area. (B) Head capsule width of each larva instar, instars followed by a different letter have significantly different size (see Table S8). (C) Pupal length (***<0.001, N.S.: no significant difference).
In both species, the head morphology of the second larval instar is as follows: (1) flat prognathous head, (2) maxillae, labial palpi and (3) spinneret are absent and mandibles are flattened plates (Movies S2 and S3, Fig. S4). These features are consistent with fluid-feeding behaviour in caterpillars19. In both species, third instar larvae show mandibles that conform to the normal lepidopteran chewing type (Movies S2 and S3, Fig. S4). This indicates that larval hypermetamorphosis in C. cecidophora occurs between the second and third instar, as in many other Caloptilia species14, but in this case, happens concurrently with the switch to feeding on induced gall tissue.
Discussion
Our results establish the existence of a leaf-mining stage during the first two larval instars of the gall-inducing micromoth C. cecidophora. This is the first example of an insect switching from leaf-mining to the induction of a complex gall. Gall-like structures have previously been described in leaf-mines but differ from what are described here because they only involve a limited formation of callus3,8. Our results provide evidence for a transitional state between leaf-miners and gall-inducers. This suggests that leaf-miners offers a third evolutionary pathway to the gall-inducing habits of insects. Price previously proposed two evolutionary pathways, one from sedentary herbivory and another from boring herbivory10.
We show that the gall stage evolves from a tentiform mine and that the induction of the gall occurs at the third instar (Fig. 5). Like most other Caloptilia species, the third instar larva draws together the spongy parenchyma of the mine edges using silk (Movie S2). However, in the case of C. cecidophora, activation of cell division at the mine edges leads to their fusion, which progressively creates a closed cavity inside the altered spongy parenchyma. The gall closure mechanism is a mix of a physical process, the lower epidermis folding involving the use of silk, and an uncharacterized chemical process, activation by the larva of leaf cell hypertrophy and proliferation through hyperplasia (Fig. 5). To our knowledge, a gall ontology that begins with mechanical folding of the leaf by the insect has never been described before in arthropod-induced galls. Furthermore, this shows that folding of a three-dimensional tentiform mine might constitute a preadaptation to gall induction, thus supporting that induction of leaf depression can precede leaf gall evolution10.
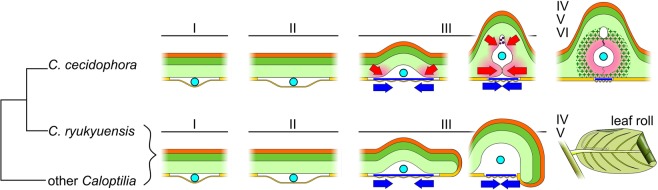
Figure 5.
Caloptilia cecidophora interaction with its host plant compared to C. ryukyuensis and other Caloptilia. Every first and second instars are epidermis miners in this genus. Like other Caloptilia, C. cecidophora third instar folds its bloch mine into a tentiform shape by weaving silk threats on lower epidermis; then, the mine folding is the result of mechanical forces (blue arrows). However, contrary to other species of the genus, C. cecidophora activates tissue growth at its feeding sites, inducing morphogenetic movements (red arrows) that progressively seal the two joined upper leaf sides. Secondary morphogenesis occurs at upper part of the larval chamber, trapping frass and creating a lacuna. Late stages of this species stay in the gall until pupation, whereas other Caloptilia leave the tentiform mine to build a leaf-roll.
The gall-inducing habit is associated with a significant reduction in the duration of the leaf-mining larval stage. In C. cecidophora the first two leaf-mining instars last only three days compared to five days for its closest relative C. ryukyuensis. The larva of C. ryukyuensis builds what appears to be a longer serpentine mine in the first instar. In contrast, the larva of C. cecidophora builds a mine whose dimensions are reduced to what appears to be the minimal surface required for the creation of a tentiform mine.
In addition, this shortening of early larval development duration noticed in C. cecidophora is associated with a difference of growth pattern in comparison to C. ryukyuensis (Fig. 4). Whereas C. ryukyuensis has a continuous growth, C. cecidophora larvae keep the same size from the first to the third instar. C. cecidophora larval growth begins when the gall starts to develop. A similar pattern is seen in gall inducing cynipids, for which larvae remain very small until putatively defensive gall morphologies have developed20. As a result, the larval growth accumulates a one-instar delay compared to C. ryukyuensis. C. cecidophora makes up for this early absence of growth by adding a sixth instar that does not occur in other Caloptilia species. This peculiar growth pattern compared to its non-gall-inducing sister species suggests an intense selective pressure to reduce the duration of the early leaf-mining stages. It supports the hypothesis that early stages of gall formation, when the insect does not benefit yet from gall protection, represent a “window of vulnerability” to enemy attacks21–23.
The internal structure of C. cecidophora galls with a layer of neovascularized nutritive tissue surrounded by sclerenchyma (Fig. 3), which confers mechanical resistance to the gall shows the ability of C. cecidophora to induce tissue differentiation. This internal organization in two layers is similar to some of the most complex galls of cynipids or cecidomyiids20. This convergent internal structure suggests that there might be limited ways of inducing galls due to plant physiological constraints.
Gall induction did not proceed after we killed the second instar leaf-mining larva. This suggests that the cecidogenous substance is only secreted from the third larval instar. This differs from other gall inducers for which gall induction begins at the start of insect’s interaction with the host plant either as a feeding larva (e.g. cecidomyiids and aphid) or as an ovipositing adult (e.g. cynipids). This particularity may represent an opportunity to better understand the mechanism of gall induction and to identify chemical effectors involved. Omics and chemical investigations are facilitated by comparative approaches24. The mixed feeding habit of C. cecidophora and its phylogenetic proximity with non-gall-inducing relatives allow such approaches. In addition, we developed a rearing protocol to produce large number of adults from eggs in greenhouses. These lab colonies can be used to set up experiments in controlled conditions, making C. cecidophora an excellent model to investigate fundamental questions about the physiology and evolution of gall induction.
Supplementary information
Acknowledgements
We thank Akihisa Hamatani and Taisuke Amano for their help for collecting, rearing and measuring insects, as well as Véronique Lainé-Prade, Takehiro Masumura and Tomoka Miyahara for preparing some samples for histology. We thank Marion Harris for insightful comments on a previous version of this manuscript. We thank Anantanarayanan Raman, Rosy dos Santos Isaias, Graham Stone, Odette Rohfritsch and especially Atsushi Kawakita for their comments and suggestions. We thank J.-C. Simon, and G. Dubreuil for fruitful discussions. This study was supported by the Projet région Centre Insecteffect to D.G., the Bilateral Joint Research Project Origen between the National Center for Scientific Research and the Japan Society for the Promotion of Science to D.G. and I.O. (project N° 1724), the JSPS KAKENHI Grant Number 17H06260 to I.O. and the strategic prioritized research in KPU to I.O. and S.T. We would like to thank the ENS Lyon for the PhD scholarship of A.G. and the doctoral school of the University of Tours for providing mobility support to A.G.
Author Contributions
I.O. conceived the idea; A.G., D.G., C.L.V. and I.O. designed the study; A.G. and I.O. conducted sampling; A.G. conducted rearing, experiments and data analysis; A.G., F.L. and S.T. conducted histological observations; A.G., D.G., C.L.V. and I.O. wrote the manuscript. All authors gave final approval for publication.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-43213-7.
References
- 1.Raman, A., Schaefer, C. W. & Withers, T. M. Biology, ecology, and evolution of gall-inducing arthropods. 1, (Science Publishers Enfield, 2005).
- 2.Meyer, J. & Maresquelle, H. J. Anatomie des galles (1983).
- 3.Guiguet A, et al. Inside the horn of plenty: Leaf-mining micromoth manipulates its host plant to obtain unending food provisioning. Plos One. 2018;13:e0209485. doi: 10.1371/journal.pone.0209485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price PW, Fernandes GW, Waring GL. Adaptive nature of insect galls. Environ. Entomol. 1987;16:15–24. doi: 10.1093/ee/16.1.15. [DOI] [Google Scholar]
- 5.Nyman T, Widmer A, Roininen H. Evolution of gall morphology and host-plant relashionships in willow-feeding sawflies (Hymenoptera: Tenthredinidae) Evolution. 2000;54:526–533. doi: 10.1111/j.0014-3820.2000.tb00055.x. [DOI] [PubMed] [Google Scholar]
- 6.Crespi BJ, Carmean DA, Chapman TW. Ecology and evolution of galling thrips and their allies. Annu. Rev. Entomol. 1997;42:51–71. doi: 10.1146/annurev.ento.42.1.51. [DOI] [PubMed] [Google Scholar]
- 7.Stern DL. Phylogenetic evidence that aphids, rather than plants, determine gall morphology. Proc R Soc Lond B. 1995;260:85–89. doi: 10.1098/rspb.1995.0063. [DOI] [Google Scholar]
- 8.Hering, E. M. Biology of the leaf miners. (Springer Science & Business Media, 1951).
- 9.Dempewolf, M. Dipteran leaf miners. in Biology, ecology, and evolution of gall-inducing arthropods (eds Raman, A., Schaefer, C. W. & Withers, T. M.) 1, 407–429 (Science Publishers Inc., 2005).
- 10.Kumata T. Descriptions of twenty new species of the genus Caloptilia Hübner from Japan including the Ryukyu Islands (Lepidoptera: Gracillariidae) Insecta Matsumurana. 1966;29:1–21. [Google Scholar]
- 11.Kumata, T., Kobayashi, S. & Hirowatari, T. Gracillariidae. Stand. Moths Jpn. IV Gakken Educ. Publ. Tokyo Jpn (2013).
- 12.Ohshima I. Techniques for continuous rearing and assessing host preference of a multivoltine leaf-mining moth, Acrocercops transecta (Lepidoptera: Gracillariidae) Entomol. Sci. 2005;8:227–228. doi: 10.1111/j.1479-8298.2005.00120.x. [DOI] [Google Scholar]
- 13.Kawakita A, Okamoto T, Goto R, Kato M. Mutualism favours higher host specificity than does antagonism in plant-herbivore interaction. Proc. R. Soc. B Biol. Sci. 2010;277:2765–2774. doi: 10.1098/rspb.2010.0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumata, T. A new stem-miner of alder in Japan, with a review of the larval transformation in the Gracillariidae (Lepidoptera) 28.
- 15.Kumata T. A new genus of Gracillariidae, with three new species from Asia (Lepidoptera) Insecta Matsumurana Ser. Entomol. New Ser. 1985;32:109–137. [Google Scholar]
- 16.Thévenaz P, Unser M. User-friendly semiautomated assembly of accurate image mosaics in microscopy. Microsc. Res. Tech. 2007;70:135–146. doi: 10.1002/jemt.20393. [DOI] [PubMed] [Google Scholar]
- 17.Rice WR. Analyzing Tables of Statistical Tests. Evolution. 1989;43:223. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- 18.Kumata T. A taxonomic revision of the Gracillaria group occuring in Japan (Lepidoptera: Incurvarioidea) Insecta Matsumurana Ser. Entomol. New Ser. 1982;26:1–186. [Google Scholar]
- 19.Body M, Burlat V, Giron D. Hypermetamorphosis in a leaf-miner allows insects to cope with a confined nutritional space. Arthropod-Plant Interact. 2015;9:75–84. doi: 10.1007/s11829-014-9349-5. [DOI] [Google Scholar]
- 20.Stone GN, Schönrogge K. The adaptive significance of insect gall morphology. Trends Ecol. Evol. 2003;18:512–522. doi: 10.1016/S0169-5347(03)00247-7. [DOI] [Google Scholar]
- 21.Cornell HV. Survivorship, Life History, and Concealment: A Comparison of Leaf Miners and Gall Formers. Am. Nat. 1990;136:581–597. doi: 10.1086/285117. [DOI] [Google Scholar]
- 22.Cornell HV. The Secondary Chemistry and Complex Morphology of Galls Formed by the Cynipinae(Hymenoptera): Why and How? Am. Midl. Nat. 1983;110:225–234. doi: 10.2307/2425263. [DOI] [Google Scholar]
- 23.Craig TP, Itami JK, Price PW. The Window of Vulnerability of a Shoot-Galling Sawfly to Attack by a Parasitoid. Ecology. 1990;71:1471–1482. doi: 10.2307/1938284. [DOI] [Google Scholar]
- 24.Oates C, Denby K, Myburg A, Slippers B, Naidoo S. Insect Gallers and Their Plant Hosts: From Omics Data to Systems Biology. Int. J. Mol. Sci. 2016;17:1891. doi: 10.3390/ijms17111891. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.