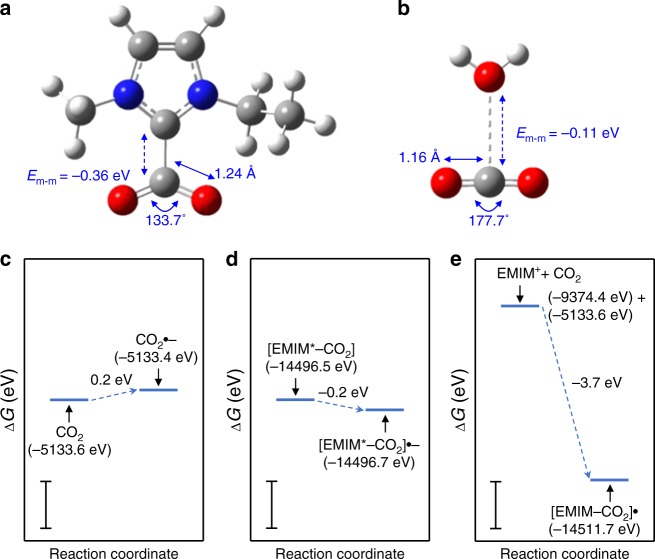
Fig. 3.
The role of the ionic liquid in Au NP-photocatalyzed CO2RR. a,b DFT-optimized geometries of [EMIM*-CO2] (a) and [H2O-CO2] (b) complexes. C, H, O, and N atoms are depicted by gray, white, red, and blue spheres, respectively. Key bond lengths, bond angles, and the energy of intermolecular interaction, Em-m, are indicated for each of the complexes. c–e DFT-computed free energy cost, ∆G, of formation of the 1e− adduct of CO2 (c), 1e− adduct of [EMIM*-CO2] (d), and 1e− adduct of CO2 in the presence of EMIM+ (e). In the latter case, the 1e− adduct of CO2, CO2•−, is stabilized by complexation with EMIM+ as described by the net process: EMIM+ + CO2 + e−→ [EMIM-CO2]•. In c–e, the free energy of each species is indicated in parentheses. Scale bars are 1 eV in length