Abstract
As the development of oxygen evolution co-catalysts (OECs) is being actively undertaken, the tailored integration of those OECs with photoanodes is expected to be a plausible avenue for achieving highly efficient solar-assisted water splitting. Here, we demonstrate that a black phosphorene (BP) layer, inserted between the OEC and BiVO4 can improve the photoelectrochemical performance of pre-optimized OEC/BiVO4 (OEC: NiOOH, MnOx, and CoOOH) systems by 1.2∼1.6-fold, while the OEC overlayer, in turn, can suppress BP self-oxidation to achieve a high durability. A photocurrent density of 4.48 mA·cm−2 at 1.23 V vs reversible hydrogen electrode (RHE) is achieved by the NiOOH/BP/BiVO4 photoanode. It is found that the intrinsic p-type BP can boost hole extraction from BiVO4 and prolong holes trapping lifetime on BiVO4 surface. This work sheds light on the design of BP-based devices for application in solar to fuel conversion, and also suggests a promising nexus between semiconductor and electrocatalyst.
Subject terms: Hydrogen energy, Solar energy, Photocatalysis, Two-dimensional materials
Photoelectrochemical water splitting affords an integrated approach for converting light to fuel, but devices typically suffer poor activities and stabilities. Here, authors incorporate black phosphorene into bismuth vanadate photoanodes to boost hole extraction and device lifetimes.
Introduction
Photoelectrochemical (PEC) water splitting on polycrystalline BiVO4 photoanodes has attracted considerable attention in recent years due to the narrow bandgap (2.4–2.5 eV) and deep valence band edge of BiVO4, which enable visible light harvesting and water oxidation1,2. However, the occurrence of surface/bulk charge recombination due to the poor charge transport characteristics and short hole-diffusion length (<70 nm) of BiVO4 leaves room to improve the PEC performance of BiVO4 photoanodes3,4. Heteroatom doping5–7, component or structural tuning8–10, and loading of oxygen evolution co-catalysts (OECs)11–14 are identified as the most promising approaches for overcoming these drawbacks and improving the PEC performance of BiVO4 photoanodes. Among these methods, OEC loading can strongly suppress surface recombination in BiVO4 photoanodes and also shift the photocurrent onset potential close to its flat-band potential for water oxidation, which is the most significant feature for achieving unbiased solar water splitting15,16.
Recently, van de Krol et al. re-stated the roles of some OECs, such as Co-Pi and RuOx, from the perspectives of the surface reaction kinetics and surface recombination. The researchers mainly pointed out that the water oxidation capability of OECs is strongly limited by their small thermodynamic driving force caused by insufficient hole extraction from the photoanodes17. This limitation means that for one of the best existing photoanode materials with an OEC, BiVO4/OEC, the band bending of BiVO4 at the electrode/electrolyte interface must be optimized18–20. For instance, Kim and Choi demonstrated that the incorporation of a FeOOH compound can accelerate hole transport from BiVO4 to the NiOOH OEC because the hole transport resistance of FeOOH is lower than that of NiOOH13. Zhong et al. suggested that the deposition of p-NiO on the CoOx OEC/BiVO4 surface to form a p–n junction interface can be beneficial for rapid hole extraction to reduce bulk charge recombination in BiVO421. Gong’s group directly employed a p-Co3O4 OEC instead of CoOx and proved that a p-type semiconductor having OEC functions can result in simultaneous enhancements in the hole extraction and water oxidation capabilities of the BiVO4 photoanode22. Therefore, promoting hole extraction from BiVO4 to OECs by improving their interface resistance still holds broad interest and significance for enhancing the PEC performance.
As a novel 2D family of materials, exfoliated black phosphorene (BP) layers that are 2–20 nm thick can show p-type semiconductor properties with high hole mobility (1000 cm2 V−3s), which are caused by the unavoidable presence of oxygen species23,24. On the other hand, its bandgap properties, which are dependent on the number of layers, result in a tunable bandgap between the bulk value of 0.3 eV to the monolayer value of 2.1 eV; therefore, BP is considered a photoabsorber of visible and near-infrared solar light for solar light harvesting25. Apart from several compelling succeeds in the application of exfoliated BP as a photocatalyst for H2 generation and water splitting26–30, employing exfoliated BP and its tailored integration with photoanodes to enhance the PEC performance for highly efficient water splitting has not been given much attention31.
In this study, we first demonstrate that the insertion of exfoliated BP nanosheets with ∼4 layers between BiVO4 photoanodes and conventional OEC layers can lead to ultra-rapid hole extraction. The electrochemical analysis reveals a built-in p/n electric field formed by the BP/BiVO4 heterostructure, in which the space-charge region results in an upward shift in the energy level of the BP nanosheets. After coating of the photoanode with an additional thin OEC layer (NiOOH), the interfacial band-edge energetics strongly drive holes from BiVO4 to the NiOOH surface for efficient water oxidation. As a result, NiOOH/BP/BiVO4 achieves a photocurrent density of 4.48 mA·cm−2 at a bias of 1.23 V vs. RHE, which is 4.2 times higher than that of pure BiVO4 and 1.5 times higher than that of NiOOH/BiVO4. Moreover, the hole extraction role of the BP nanosheets is successfully evidenced by two other OECs (MnOx and CoOOH), demonstrating the potential of BP as an auxiliary to enhance water oxidation.
Results
Characterization of the BP/BiVO4 photoanode
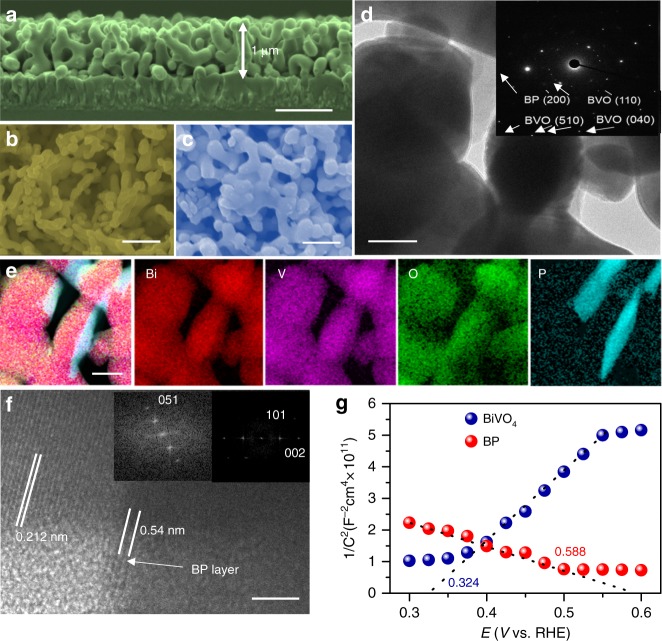
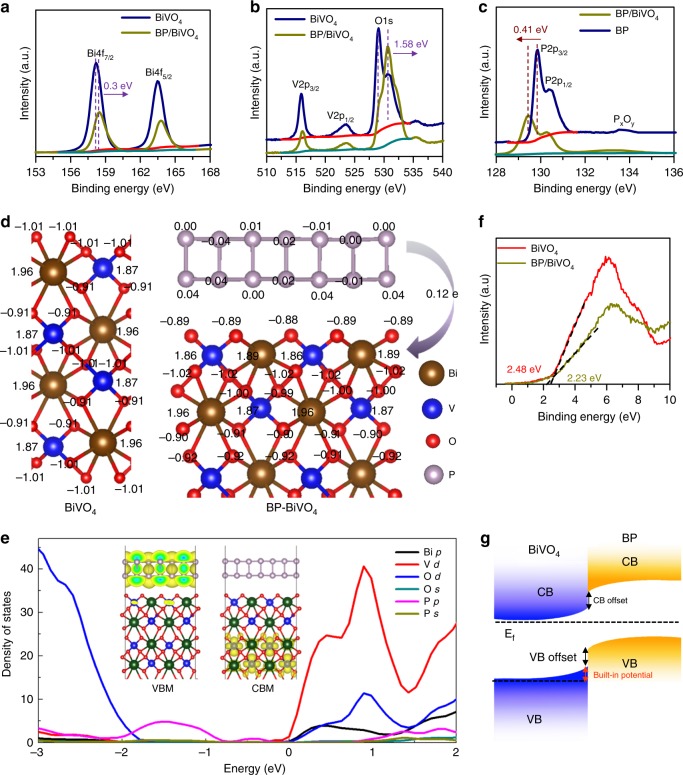
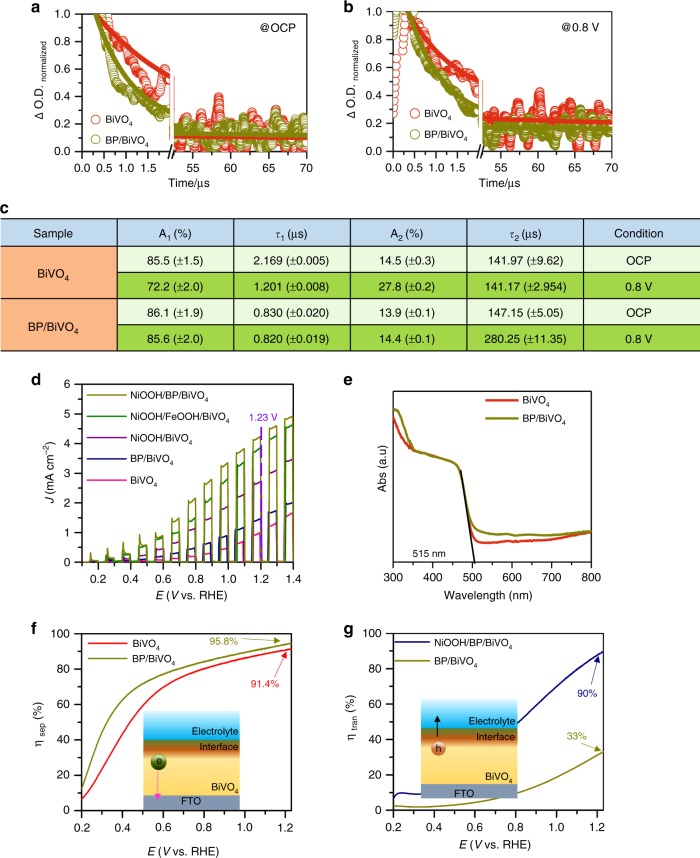
BP nanosheets were synthesized by liquid exfoliation of bulk BP particles and dispersed in isopropanol (IPA) under an N2 atmosphere (Supplementary Fig. 1). The redshifted Raman signals of the BP nanosheets confirm the successful exfoliation of bulk BP (Supplementary Fig. 2). The atomic force microscopy (AFM) image of the exfoliated BP nanosheet layers shows a distinct 2D morphology with an average thickness of ∼2.2 nm, corresponding to 4 layers (Supplementary Fig. 3)32. High-resolution transmission electron microscopy (HR-TEM) images of the exfoliated BP nanosheets display clear lattice fringes with a d-spacing of 0.34 nm, corresponding to the (040) plane (Supplementary Fig. 4). A nanoporous BiVO4 photoanode was fabricated by using an electro-deposited BiOI film as a precursor based on the previous method13. The thickness of the as-prepared BiVO4 photoanode was ca. 1 µm (Fig. 1a). Considering that the lateral size of BP is larger than the pore size of BiVO4 film, the depositing BP on BiVO4 photoanode was assisted by centrifuge-coated method (See experimental section for detail). Compared to the morphology of the pure BiVO4 photoanode (Fig. 1b and Supplementary Fig. 5a), the SEM image of BP/BiVO4 does not reveal the presence of BP nanosheets on surface of BiVO4 photoanode (Fig. 1c and Supplementary Fig. 5b), which is in stark contrast to that the BiVO4 photoanode is immersed into the BP dispersion by natural adsorption or deposition (Supplementary Fig. 6). X-ray diffraction (XRD) analysis demonstrates monoclinic BiVO4 crystal, which remains unchanged after the deposition of BP, but a small diffraction peak of BP can be detected (Supplementary Fig. 7). However, although the observation on BP/BiVO4 by TEM image could not distinguish the presence of BP nanosheets (Fig. 1d), the electron diffraction spot confirms the co-existence of poly-crystalline BiVO4 and BP components (insert in Fig. 1d). High-angle annular dark-field scanning TEM-energy-dispersive spectroscopy (HAADF-STEM-EDX) reveals an obvious sheet-like distribution pattern of P element clinging to BiVO4 particles (Fig. 1e). Further enlarged HAADF-STEM image exhibits a bright area on the BiVO4 particle, which can be identified as a BP sheet (Supplementary Fig. 8). However, the structure incompatibility between the 2D BP sheets and 3D BiVO4 nanopores may lead to uncovered part of BiVO4 by BP. Nevertheless, its high-resolution TEM (HR-TEM) image shows the distinct interface of BP/BiVO4, in which the lattice spacing of 0.212 nm corresponds to the (051) planes of monoclinic BiVO414, while the other lattice spacing of 0.54 nm is consistent with the interlayer distance of BP along the c-axis33. Compared to the BP/BiVO4 prepared by the centrifuge-coated method, the naturally deposited BP/BiVO4 shows the poor connection between the BP sheet and BiVO4 particles (Supplementary Fig. 9). The conductivity of the BiVO4 photoanode and BP nanosheets was investigated with Mott-Schottky plots. As shown in Fig. 1g, BiVO4 is a typical n-type semiconductor with a Fermi energy of 0.324 V vs NHE; the n-type behaviour is usually caused by the presence of oxygen defects1. In contrast, the straight line of exfoliated BP displays a negative slope with a Fermi level of 0.588 V vs NHE, indicating p-type conductivity. The intimate contact between BiVO4 and the BP nanosheets makes it easy to construct an electric field from the p/n junctions. High-resolution X-ray photoelectron spectroscopy (XPS) of Bi, O, and V can determine the built-in potential and band offsets at the interface34. As shown in the Bi 4f (Fig. 2a) and O 1s/V 2p (Fig. 2b) XPS spectra, the Bi 4f7/2 core-level and O 2p core-level spectra of BP/BiVO4 shift by 0.3 and 1.58 eV, respectively, to higher binding energies in comparison to those of pure BiVO4, whereas the V 2p3/2 core-level spectra of BP/BiVO4 and BiVO4 are almost the same. Accordingly, the binding energy of the P 2p core-level spectrum of BP shifts by ∼0.41 eV to lower energy after integration with BiVO4 (Fig. 2c). The differential charge density diagram of the BP/BiVO4 heterointerface was compared with that of the clean BiVO4 surface by Bader charge analysis, which can further reveal the charge transfer direction (Fig. 2d). In detail, BP with a 1 × 3 supercell donates 0.12 e to BiVO4 with a 1 × 2 supercell, and the transferred charges are mainly distributed on the interfacial O atoms with negligible effect on the V atoms. Density of states (DOS) calculations were then conducted to determine the electronic structure of BiVO4. The calculated valance band (VB) maximum and conduction band (CB) minimum of monoclinic BiVO4 with a standard space group of C2/c is mainly comprised of O 2p and V 3d orbitals (Supplementary Fig. 10), which is in good agreement with a previous report35. Remarkably, BP profoundly influences the VB electronic structure of BiVO4 through the overlap of the P 2p and O 2p orbitals independent of the CB minimum of BiVO4 (Fig. 2e). As the O 2p and V 3d orbitals contribute to the VB of BiVO4, the charge transfer that occurs on the O and V atoms at the p/n junction might be implicated in upward bending of the VB. As shown in Fig. 2f, The VB position is shifted upward from 2.48 eV for BiVO4 to 2.23 eV for BP/BiVO4, implying that the positrons (holes) are the dominant carriers across the p/n junction under reverse bias36. According to the above experimental and theoretical results, Fig. 2g summarizes the possible band offsets and built-in potential of the BP/BiVO4 heterojunction. Since the BP/BiVO4 photoanode performs under external bias to facilitate electron transport from BP/BiVO4 to the counter electrode in PEC water splitting, the external bias is therefore regarded as reverse bias. Positrons as the dominant carriers across the BP/BiVO4 heterointerface would promote hole extraction from BiVO4 to BP under external bias. To determine the hole extraction as a function of applied bias, the in-situ ultrafast transient absorption (TA) spectroscopy is performed to evaluate the hole trapping behaviours. As reported previously, the TA signal of surface-trapped holes for BP/BiVO4 hybrid is mainly located in the wavelength ranging from 400 to 700 nm27. Therefore, the TA signal at 500 nm for BiVO4 and BP/BiVO4 anodes, respectively under open circuit potential and 0.8 V vs. Ag/AgCl in 0.5 M phosphate buffer (KPi, pH 7.1) are monitored. Compared with BiVO4 anode, the intensity of the absorption signal for BP/BiVO4 anode is significantly increased as anodic shifting the applied bias (Supplementary Fig. 11). In Fig. 3a, b, the decay signal was further fitted to a biexponential decay model with a fast component (τ1) and a slow component (τ2)37,38, and the lifetimes are summarized in Fig. 3c. Since the fast component, τ1, is associated with the hole being trapped at near band edge, the decreased τ1 values for both BiVO4 and BP/BiVO4 photoanodes under applied bias can be considered the external bias that boosts hole transport37,38. The BiVO4 photoanode shows similar τ2 value with 141.97 µs, 14.5% under OCP and 141.17 µs, 27.8% at 0.8 V bias, whereas the BP/BiVO4 anode exhibits an approximate two-fold increase in τ2 value from 147.15, 13.9% under OCP to 280.25 µs, 14.4% at 0.8 V bias. The slow component, τ2, can be ascribed to the holes being trapped at photoanode/electrolyte interface, which is expected to yield long-lived holes for water oxidation37. For BP/BiVO4 anode, the two-fold increase in slow component suggests that the increased number of holes are extracted from BiVO4 to photoanode/electrolyte interface under applied bias, which is consistent with our assumption from their band alignment.
Fig. 1.
Morphology and conductivity characterizations. a Cross-section SEM image, scan bar: 1 μm. b Top view SEM images of as-prepared BiVO4 photoanode and (c) the top view SEM image of BP/BiVO4 photoanode, scan bar: 500 nm. d TEM image of BP/BiVO4 photoanode, scan bar: 100 nm, insert is electron diffraction spot. e HAADF-STEM-EDX element mapping and f HR-TEM image of BP/BiVO4 heterojunction, scan bar: 5 nm. g Mott-Schottky plots of BP and BiVO4 electrodes measured with a frequency of 500 Hz and amplitude of 10 mV in KPi electrolyte (pH = 7.1)
Fig. 2.
Electronic structure characterizations. a Bi 4f, (b) V 2p and O 1s XPS of BiVO4 and BP/BiVO4 photoanodes, (c) P 2p XPS of BP and BP/BiVO4 photoanode, d The charge density difference between BiVO4 and BP/BiVO4, (e) VB XPS of BiVO4 and BP/BiVO4 photoanodes, (f) DOS of BP/BiVO4 heterojunction, (g) Energy diagram of BP/BiVO4 heterojunction interface
Fig. 3.
In-situ TA spectroscopic evidence for hole extraction as a function of applied bias. The decay recorded at 500 nm under OCP and 0.8 V vs Ag/AgCl in 0.5 M KPi electrolyte (pH = 7.1) was fitted to a biexponential decay model, for (a) BiVO4 and (b) BP/BiVO4. c Fitting parameters of the TA signals. PEC performance, optical properties and electrochemical characterizations. d Chopped J–V curves of various photoanodes in KPi electrolyte (pH = 7.1) under AM 1.5 illumination. e UV–Vis absorbance of BiVO4 and BP/BiVO4 photoanodes. f Charge separation efficiencies of BiVO4 and BP/BiVO4 photoanodes. g Charge transfer efficiencies of BP/BiVO4 and NiOOH/BP/BiVO4 photoanodes
PEC Performance Evaluation of BP/BiVO4 anode
The improvement in the PEC performance of BiVO4 imparted by the p–n junction formed with BP was investigated by measuring the J–V curves in 0.5 M phosphate buffer (KPi, pH 7.1) from rear side illumination (AM 1.5G, 100 mW cm−2). As shown in Fig. 3d, an obvious improvement in the photocurrent density of BiVO4 is observed in the presence of BP, whereas the dark current density demonstrates that the water oxidation kinetics are slower for BP/BiVO4 than for BiVO4 (Supplementary Fig. 12). These results imply that the enhanced photocurrent density of BP/BiVO4 does not originate from surface water oxidation. As a consequence, a NiOOH layer that is a well-defined OEC for water oxidation was electro-deposited on the BP/BiVO4 electrode to enhance the water oxidation kinetics (Supplementary Fig. 13)39. SEM and TEM images of NiOOH/BP/BiVO4 reveal a near-surface BP nanosheet layer buried by an amorphous NiOOH layer (Supplementary Fig. 14). Remarkably, the NiOOH/BP/BiVO4 photoanode achieves a photocurrent density of 4.48 mA cm−2 at 1.23 V vs NHE, which is 1.5-fold higher than that of the NiOOH/BiVO4 photoanode (3.03 mA·cm−2 at 1.23 V vs. NHE) and 2.7-fold higher than that of the BP/BiVO4 photoanode (1.66 mA cm−2 at 1.23 V vs NHE). As reported by Kim and Choi13, the interfacial resistance between BiVO4 and NiOOH creates an energy barrier that impedes rapid hole transfer to the reaction surface, so the insertion of a FeOOH layer between NiOOH and BiVO4 facilitates hole transfer, hence enhancing the PEC performance. In our case, the NiOOH/FeOOH/BiVO4 photoanode exhibits a photocurrent density of 4.13 mA·cm−2 at 1.23 V vs NHE, which is lower than the photocurrent density of NiOOH/BP/BiVO4. In contrast, the FeOOH/BiVO4 photoanode exhibits a higher photocurrent density than BP/BiVO4 (Supplementary Fig. 15). These results clearly illustrate that the superior PEC performance of the NiOOH/BP/BiVO4 photoanode relative to that of NiOOH/FeOOH/BiVO4 results from the interfacial behaviour associated with hole transfer. To understand how hole transfer was improved by the presence of BP nanosheets, the charge separation efficiency (ηsep) was calculated by the following equation40:
| 1 |
where JHS is the photocurrent density measured in a hole scavenger-containing electrolyte and Jabs is associated with the maximum photocurrent density (Jmax) and light harvesting efficiency (LHE). The UV–Vis absorption spectra of BiVO4 and BP/BiVO4 are shown in Fig. 3e. The spectrum of BP/BiVO4 contains the unchanged absorption edge of BiVO4 at 515 nm in addition to a longer absorption tail up to 800 nm. The expanded absorption region of BP/BiVO4 can be ascribed to the narrower bandgap of BP (Supplementary Fig. 16). However, the BP nanosheets might not act as an efficient photosensitizer to inject electrons, as the BP/BiVO4 photoanode does not show a detectable photocurrent response at 520 nm in the presence of Na2SO3 as a hole scavenger (Supplementary Fig. 17). As a result, Jmax and LHE can be established based on only the absorption of BiVO4 ranging from 300 to 515 nm. The LHE and calculated Jmax value are shown in Supplementary Fig. 18 and the JHS is measured in the presence of Na2SO3 as a hole scavenger (Supplementary Fig. 19). The corresponding ηsep value is shown in Fig. 3f. The BP nanosheets clearly significantly improve the ηsep of BiVO4 in the entire voltage region. Excluding the possibility of electron injection by the excited BP layer, the enhanced ηsep directly points to efficient hole extraction from the excited BiVO4 photoanode to the BP layer. Nevertheless, due to its poor water oxidation ability (Supplementary Fig. 12), the charge transfer efficiency (ηtran) of the BP/BiVO4 photoanode, which is calculated as JPh/JHS (JPh is the photocurrent density measured in KPi electrolyte)41, is lower than that of the BiVO4 photoanode (Supplementary Fig. 20). To understand the charge separation and transfer limitation, the J–V curves of BiVO4 and BP/BiVO4 photoanodes were measured from front illumination. As shown in Supplementary Fig. 21a, the photocurrent densities of BP/BiVO4 photoanode are still better than that of BiVO4 in the KPi electrolyte with and without hole scavenger. The calculated ηsep is similar to the result obtained by rear illumination (Supplementary Fig. 21b), whereas the calculated ηtran (Supplementary Fig. 21c) display different tendency from rear illumination. The enhanced ηtran of BP/BiVO4 photoanode from front illumination is unexpected, which might be ascribed to the effect of surface passivation by BP layers on the reduction of surface recombination that compensates poor water oxidation ability42. Therefore, the subsequent introduction of the NiOOH overlayer on BP/BiVO4 is crucial for improving the hole capability towards efficient water oxidation. ηtran of NiOOH/BP/BiVO4 (90%) is ∼2.7 times higher than that of BP/BiVO4, which has a value of 1.23 V vs RHE (Supplementary Fig. 22 and Fig. 3g), indicating the occurrence of strong synergistic effects between NiOOH and BP.
Hole Extraction Behaviour of BP Nanosheets
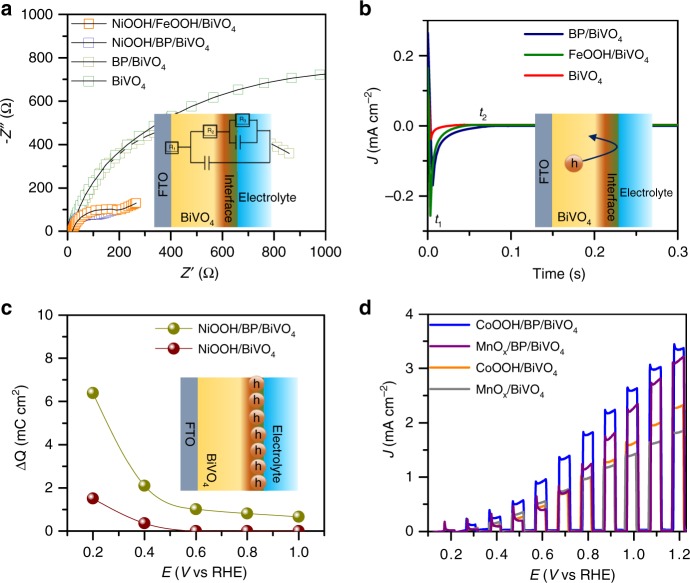
The capability of BP to perform hole extraction is further proven by electrochemical impedance spectroscopy (EIS) conducted at 0.6 V vs RHE in KPi electrolyte (Fig. 4a). The Nyquist plots were fitted by an equivalent circuit, as shown in the inset of Fig. 4a and the results are displayed in Supplementary Table 1. The NiOOH overlayer clearly significantly reduces the charge transfer resistance (R2) of both FeOOH/BiVO4 and BP/BiVO4 due to the enhanced water oxidation capability, while NiOOH/BP/BiVO4 possesses a smaller charge transport resistance (R2) than NiOOH/FeOOH/BiVO441. Based on the smaller charge transport resistance of BP/BiVO4 relative to that of BiVO4, the results clearly show that the hole extraction behaviour of the BP nanosheets is superior to that of FeOOH. Furthermore, the bulk capacitances (Cbulk) for all photoanodes are almost same, which can be ascribed to the redox process of V4+/V5+ 19,41. The capacitances at the electrode/electrolyte interface for BP/BiVO4, NiOOH/FeOOH/BiVO4 and NiOOH/BP/BiVO4 are significantly increased, which can be related to the surface layer that modifies surface state of BiVO443. Figure 4b demonstrates the occurrence of long-lived hole storage by the BP nanosheet layer based on analysis of the transient cathodic current. t1, corresponding to photocurrent quenching under instantaneous light-off conditions, gradually decays to a steady state (t2), thus showing a cathodic current. The delay in the steady-state cathodic current indicates that the separated holes that reach the electrode/electrolyte interface are not involved in water oxidation but are instead stored at the electrode surface. Therefore, the large value of t2-t1 for BP/BiVO4 indicates the presence of long-lived holes at the surface of BiVO444–46. The charge storage behaviour of the NiOOH/BP/BiVO4 photoanode against an applied bias can be calculated from the transient-state photocurrent based on the chronoamperometry curve measured under chopped illumination and linear sweep voltammetry (LSV) curves, respectively (Supplementary Fig. 23). The photocurrent drop from the transient state to the steady state can be ascribed to the number of holes stored43. Compared to the NiOOH/BiVO4 photoanode, the number of holes stored by the NiOOH/BP/BiVO4 photoanode is obviously higher across the entire potential region, especially at low bias (Fig. 4c). The fate of the holes extracted is to reach the surface, then participates in the water oxidation reaction at high potentials, where the injection barrier no longer impedes the charge transfer from the electrode to the electrolyte. These results further demonstrate the strong capability of the BP nanosheet layer to perform hole extraction towards water oxidation occurring at surface of OECs.
Fig. 4.
PEC performance and electrochemical characterizations. a EIS Nyquist plots measured at 0.6 V vs RHE in KPi electrolyte (pH = 7.1) under AM 1.5 illumination, b Delay of the cathodic photocurrent curves measured at 0.2 V vs RHE in KPi electrolyte (pH = 7.1) under AM 1.5 illumination, c Charge storage capability against applied bias for both NiOOH/BiVO4 and NiOOH/BP/BiVO4 photoanodes. d Chopped J–V curves of CoOOH/BP/BiVO4 and MnOx/BP/BiVO4 photoanodes in KPi electrolyte (pH = 7.1) under AM 1.5 illumination
To further illustrate the impressive role of the BP nanosheet layer in hole extraction, two other well-defined OECs, CoOOH, and MnOx, were respectively spin-coated and photo-deposited on the BP/BiVO4 photoanode surface (Supplementary Fig. 24). The enhancement in the PEC performance is evidenced by comparison of the catalysts deposited on BiVO4 photoanodes, as demonstrated by the cyclic voltammetry (CV) curves in Fig. 4d. The enhancement factors induced by the BP layer are summarized in Supplementary Table 2, in which an average 1.5-fold enhancement is observed, and the NiOOH OEC overlayer demonstrates the highest PEC performance, which arises from its superior water oxidation capability (Supplementary Fig. 25).
PEC Water Splitting of NiOOH/BP/BiVO4 anode
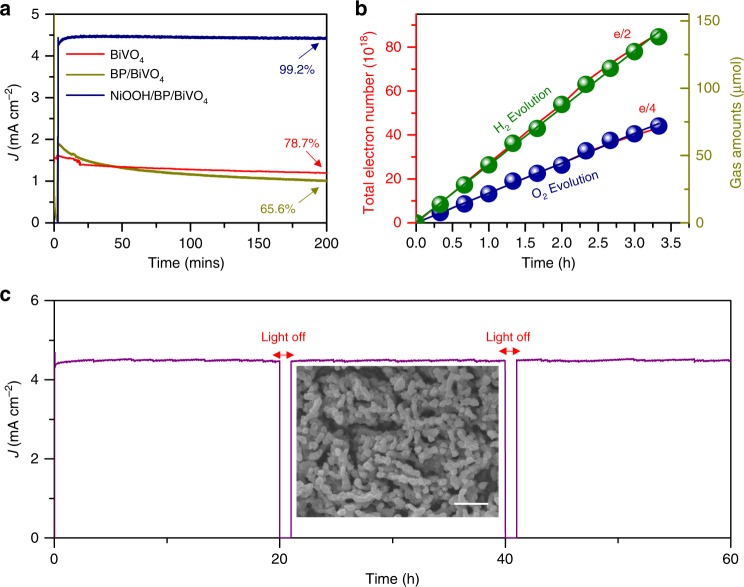
The BP nanosheet layer buried underneath the NiOOH layer exhibits a current density of 4.46 mA cm−2 at 1.23 V vs NHE for at least 200 min, as shown in Fig. 5a. Without the outmost NiOOH layer or with pure BiVO4 (BP/BiVO4 or BiVO4 photoanode), the steady-state current density at 1.23 V vs NHE gradually drops. The fading of the photocurrent density can be ascribed to anodic photocorrosion of BiVO4 by the surface-accumulated holes that arise from an insufficient water oxidation capability47,48. In addition, the oxygen gas surrounding BP may cause its self-oxidation, as determined by P 2p XPS analysis (Supplementary Fig. 26). Moreover, BiVO4 combined with BP and NiOOH layers demonstrates superior PEC performance relative to other OEC/BiVO4 photoanodes and competitive values with those improved OEC/BiVO4 photoanodes (Supplementary Table 3). The gas evolved from the NiOOH/BP/BiVO4 photoanode was measured from the photocurrent density at 1.23 V vs RHE. As shown in Fig. 5b, the linear fitting plots of both H2 and O2 nearly overlap with the theoretical number of electrons. The excellent Faradic efficiency for O2 evolution indicates that the hole-storing behaviour of BP does not impede the oxygen evolution reaction taking place at the NiOOH surface.
Fig. 5.
a Photocurrent density stability measured at 1.23 V vs RHE in KPi electrolyte (pH = 7.1) under AM 1.5 illumination. b Plots of the theoretical charge number obtained from the J–t curves collected at 1.23 V vs. RHE and the actual quantities of H2 and O2 evolution in KPi electrolyte (pH = 7.1) under AM 1.5 illumination. c Long-term stability of the NiOOH/BP/BiVO4 photoanode at 1.23 V vs RHE in KPi electrolyte (pH = 7.1) under AM 1.5 illumination, insert is SEM image after long-term testing, scan bar: 1 μm
Furthermore, the long-term durability of the NiOOH/BP/BiVO4 photoanode was investigated at 1.23 V vs RHE. This test was performed for 60 h and rested for 2 h with an interval of 20 h. As shown in Fig. 5c, the photocurrent density of the NiOOH/BP/BiVO4 photoanode is stable with a slight fluctuation between 4.31 and 4.56 mA/cm2. The morphology after testing is well-maintained (Insert in Fig. 5c). The result is a sharp contrast to the CoOOH/BP/BiVO4 photoanode which shows a rapid decrease in photocurrent density after continuous testing of 2 h (Supplementary Fig. 27). The XPS results of NiOOH/BP/BiVO4 photoanodes before and after long-term testing are shown in Supplementary Fig. 28, which indicates that the BP in the NiOOH/BP/BiVO4 photoanode is oxidized to a lesser extent. However, the BP in the CoOOH/BP/BiVO4 is almost completely oxidized (Supplementary Fig. 29). It is a fact that the BP is able to be slowly oxidized during PEC testing, whereas the electro-deposited NiOOH is believed to have conformal coverage which impedes the oxidization of BP.
Discussion
In this study, we have demonstrated that a layer of BP nanosheets can serve as an excellent hole extraction layer in a BiVO4/OEC photoanode for solar water splitting. The BP nanosheets, which were exfoliated from layered bulk BP, had the unique merit of p-type conductivity, hence enabling the formation of a p/n heterojunction with BiVO4, which facilitated hole transfer from BiVO4 to the OEC surface. As a result, the NiOOH/BP/BiVO4 photoanode exhibited a photocurrent density of 4.48 mA·cm−2 at 1.23 V vs RHE under AM 1.5 illumination, which was 4.2 times higher than that of pure BiVO4 and 1.5 times higher than that of NiOOH/BiVO4. The BP layer was found to store separated holes and then transfer them to the OEC surface, and this impressive function was universal for other OECs, such as CoOOH and MnOx. Moreover, the burying of the BP nanosheets by the OEC layer alleviated self-oxidation, thereby prolonging the stability of photoelectrochemical water splitting by BiVO4. Our work shows the potential for application of BP in solar energy conversion devices, nevertheless, a uniform coating of BP on photoanodes with strongly coupled interface is still desired for further optimization.
Methods
Materials
All chemical reagents were purchased from Aldrich without further purification. FTO was purchased from TEC-8, Pilkilton with a resistance of 14 Ω. BP crystal was purchased from Mukenano Co. LTD.
Synthesis of BP/BiVO4 Photoanodes
BiVO4 electrodes were prepared based on Lee and Chio’s method13. Bulk BP crystals were exfoliated by ultrasonication in a mixture of γ-butyrolactone (GBL) and IPA. Briefly, 20 mg of BP crystals was dispersed into 20 mL of the mixture and sonicated for 10 h at 300 W. The resultant dispersion was centrifuged at 2000 rpm for 60 min The exfoliated BP sheets were dispersed again in IPA at a concentration of 0.02 mg/mL and stored under flowing N2. The as-prepared BiVO4 photoanode with a size of 1 × 2 cm2 was placed against the wall of a 50 mL centrifuge tube with the sample side facing up. Then, 50 mL BP/IPA dispersion was added to the centrifuge tube and centrifuged by 1000 rpm for 1 min For self-absorption or deposition of BP on BiVO4 photoanode, the as-prepared BiVO4 photoanode with a size of 1 × 2 cm2 was immersed in 10 mL of the BP sheet dispersion for 2 h in a glovebox. All BP/BiVO4 photoanode was further dried at 50 ℃ in a vacuum oven.
Synthesis of OEC/BP/BiVO4 photoanodes
The NiOOH and FeOOH layers were photoelectrodeposited on photoanodes by Lee and Chio’s method13. Briefly, the NiOOH was photoelectrondeposited on BP/BiVO4 and BiVO4 photoanodes in a 0.1 M NiSO4 solution with pH adjusted to 6.8 by carefully adding NaOH at 0.11 V vs. Ag/AgCl (total charge 22 mC cm−2) under AM 1.5 illumination. The FeOOH was photoelectrodeposited on BiVO4 photoanode in a 0.1 M FeSO4 solution with gently stirring at 0.25 V vs. Ag/AgCl. The CoOOH layer was deposited on BP/BiVO4 and BiVO4 photoanodes by a spin coating method. The CoOOH ink was synthesis based on Huang’s report49. Briefly, 15 mg CoCl2·6H2O was dissolved into 40 mL ethylene glycol, and the pH value of the solution was adjusted to 9.5 by slowly dropping 25% NH3·H2O. The mixture was then transferred to a Teflon-lined stainless steel autoclave with a total 60 mL capacity and maintained at 130 °C for 24 h. The obtained α-Co(OH)2 nanosheets were dispersed in water/ethanol mixture with a 1:1 volume ratio after being washed with deionized water and ethanol several times. The pH value of greenish Co(OH)2 suspension was adjusted to 12 by adding 0.5 M NaOH solution. Then, a 5.2 wt% NaClO solution was slowly dropped into the suspension under vigorous stirring until the color changed to brown-black. The resulted CoOOH were obtained by ultrasonication assisted exfoliation for 12 h and was dispersed in alcohol to form a homogenous ink. CoOOH ink was then spin-coated onto a BiVO4 electrode at 2000 r.p.m. for 1 min and dried at 50 °C vacuum oven. The MnOx layer was photo-deposited on BP/BiVO4 and BiVO4 photoanodes based on Li’s report50. 5 mL 0.01 M MnSO4 solution and 5 mL 0.02 M NaIO3 solution were mixed in petri dish. BP/BiVO4 and BiVO4 photoanodes were placed in the above solution with the material side facing up under AM 1.5 illumination for 5 min Before PEC testing, the samples were under irradiation for 1–10 min, which can make open-circuit voltage achieve the best effect14.
Material Characterization
SEM images of the products were recorded on a field‐emission scanning electron microscope (JSM-7000F, Japan). The XRD patterns were obtained with a D500/5000 diffractometer operated in Bragg–Brentano geometry and equipped with a Cu-Kα radiation source. The HR-TEM observations were performed on a JEOL JEM-AFM 200F (Japan) electron microscope with (Cs-corrected/energy-dispersive X-ray spectroscopy (EDS)/EELS). The VB-XPS and XPS measurements were performed on an auger electron spectroscopy (AES) XPS instrument (ESCA2000 from VG Microtech in England) equipped with an aluminum anode (Al Kα, λ = 1486.6 eV). The UV–Vis DRS spectra were recorded using a UV–Vis spectrophotometer (Shimadzu UV-2550). Raman spectra were measured by using LabRam Aramis equipment (Horriba Jovin Yvon Inc., US). The AFM was measured by using Bruker Multimodel-8 equipment.
In-situ time-resolved transient absorption spectroscopy
The Laser flash photoelectrochemical water splitting measurements with transmission detection were performed with the third harmonic of the Nd;YAG laser (10Hz, NT342A, EKSPLA, 355 nm (3.5 mJ/pulse)) as excitation source and the Xe lamp (continuous wave, 300W, Newport) as the probe light source, in a three electrode system with working electrode (3 × 3 cm2), Pt counter electrode and Ag@AgCl reference electrode, and N2 saturated 0.5 M KPi buffer electrolyte. The transmitted probe light was focused on a monochromator (Princeton Instruments, Acton SpectraPro SP-2300). The output of the monochromator was monitored using a photomultiplier Tube (PDS-1, Dongwoo Optron). The transient signals were passed through an amplifier (SR445A, Stanford Research Systems) and then recorded by a digital oscilloscope (350MHz, MDO4034C, Tektronix). Photoanodes were placed in a sealed reactor with Argon purged phosphate buffer electrolyte. The applied bias was controlled with a PGSTAT204 potentiostat (Metro Autolab).
Computational method
Density functional theory calculations are performed using the plane-wave basis sets in VASP code51. The ion–electron interaction is treated by the projected-augmented wave (PAW) approximation52. The exchange-correlation functional is expressed with generalized gradient approximation Perdew−Burke−Ernzerhof (PBE-GGA)52. The energy cutoff for the plane-wave basis is set to 400 eV and the convergence threshold is set as 10−4 eV in energy and 0.01 eVÅ−1 in force. DFT+D2 method is adopted to describe the Grimme vdw correction during structure simulation and electronic calculation53. The Brillouin zone is set to 6 × 3 × 6 for bulk structure geometry optimization, 4 × 2 × 1 for BiVO4/BP heterojunctions and 12 × 6 × 1 for electronic properties. The isosurface value for VBM and CBM is set to 0.002 e/Bohr3.
PEC Measurements
The PEC performance was measured using a potentiostat (CH Instruments, CHI 660) in a three-electrode optical O-ring cell (0.37 cm2) with a Pt foil counter electrode and a saturated Ag/AgCl reference electrode (in 3 M KCl) under AM 1.5G simulated solar light illumination (100 mW cm−2) from a 150 W xenon lamp solar simulator (PEC‐L01, PECCELL, Yokohama, Japan), all electrodes were illuminated from rear side. For comparison purpose, the front-illuminated performances were measured in Quartz reactor with an active area of 0.8 cm2. In addition, Before the measurements, the solar simulator intensity was calibrated with a reference silicon solar cell (VLSI standards, Oriel P/N 91150 V). KH2PO4 and K2HPO4 buffer solution (pH = 7.1) with 0.5 M concertation was used as the electrolyte. The conversion between the potentials vs. Ag/AgCl and vs. RHE was performed using the following equations:
| 2 |
| 3 |
Prior to the PEC measurements, the electrolyte was purged with N2 to remove dissolved oxygen. In a typical J–V measurement, linear sweep voltammetry was conducted at a scan rate of 20 mV s−1. The potentiostatic mode was used to measure the electrochemical impedance spectra (EIS) with an AC voltage amplitude of 5 mV and a frequency range of 0.01–100 kHz under AM 1.5G illumination. When doing the record, a silver paste was painted on the top to increase the conductivity and an aperture was used to determine the contact area between the samples and the electrolyte.
The gas evolution was carried out in a quartz reactor, which was sealed with rubber plugs and Parafilm. The electrode (1.5 cm2) was immersed in the electrolyte in a three-electrode configuration with a 1.23 V vs RHE. Prior to the reaction and the sealing process, the electrolyte was purged with N2 gas. 1 mL of gas was analyzed by gas chromatography (Agilent Technologies 7890A GC system, USA) using a 5 Å molecular sieve column and Ar as the carrier gas. The experimental error for the evolution of H2 and O2 was considered to be ≈3%.
The theoretical electron number as a function of the J–t curve was calculated on the basis of an area of 1.5 cm2.
| 4 |
The photocurrent-to-H2 conversion efficiency and photocurrent-to-O2 conversion efficiency were determined on the basis of their linear slopes (i.e., for the photocurrent-to-H2 conversion efficiency and for the photocurrent-to-O2 conversion efficiency).
Calculation of the theoretical photocurrent in BiVO4 photoanodes
The single photon energy is calculated from Eq. (5)
| 5 |
where E(λ) is the photon energy (J), h is Planck’s constant (6.626 × 10−34 Js), C is the speed of light (3 × 108 m s−1) and λ is the photon wavelength (nm).
The solar photon flux is then calculated according to Eq. (6)
| 6 |
where Flux(λ) is the solar photon flux (m−2 s−1 nm−1), and P(λ) is the solar power flux (W m−2 nm−1).1 The theoretical maximum photocurrent density under solar illumination (AM1.5), Jmax (A m−2), is then calculated by integrating the solar photon flux between 300 to 515 nm, shown in Eq. (7):
| 7 |
where e is the elementary charge (1.602 × 10−19 C). The theoretical photocurrent of such BiVO4 photoanodes is accordingly calculated to be 6.87 mA cm−2 based our solar spectra.
Supplementary information
Acknowledgements
This work was supported by NSFC (51802157, 5151101197, 61725402), the Natural Science Foundation of Jiangsu Province of China (BK20180493), NRF Korea (NRF-2019R1A2C3010479, 2015M1A2A2074663, 2016M3D3A1A01913254 (C1 Gas Refinery Program)), the Fundamental Research Funds for the Central Universities (Nos. 30917011202, 30915012205, 30916015106), and PAPD of Jiangsu Higher Education Institutions. K.Z. acknowledges the support by “the Fundamental Research Funds for the Central Universities”, No.30918011106. K.Z. and B.J.J. contributed equally to this work.
Author contributions
K.Z. and J.H.P. conceived and designed the experiments. K.Z., B.J.J., and Y.J.C. carried out materials synthesis and electrochemical characterization. X.F.S. participated in part of the synthesis. X.J.S. participated in part of the materials synthesis. C.P. and W. Kim carried out TA measurement and analysis. S.L.Z. carried out theoretical simulation. K.Z. H.B.Z. and J.H.P. co-wrote the paper. All authors discussed the results and commented on the manuscript.
Data availability
The data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Journal peer review information: Nature Communications would like to thank Baoxue Zhou and the other, anonymous, reviewer(s) for their contributions to the peer review of this work. Peer review reports are available.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kan Zhang, Email: zhangkan@njust.edu.cn.
Haibo Zeng, Email: zeng.haibo@njust.edu.cn.
Jong Hyeok Park, Email: lutts@yonsei.ac.kr.
Supplementary information
Supplementary Information accompanies this paper at 10.1038/s41467-019-10034-1.
References
- 1.Park Y, McDonald KJ, Choi KS. Progress in bismuth vanadate photoanodes for use in solar water oxidation. Chem. Soc. Rev. 2013;42:2321–2337. doi: 10.1039/C2CS35260E. [DOI] [PubMed] [Google Scholar]
- 2.Seo JS, Nishiyama H, Yamada T, Domen K. Visible-light-responsive photoanodes for highly active, stable. Water Oxid. Angew. Chem. Inter. Ed. 2018;57:8396–8415. doi: 10.1002/anie.201710873. [DOI] [PubMed] [Google Scholar]
- 3.Tan HL, Amal R, Ng YH. Alternative strategies in improving the photocatalytic and photoelectrochemical activities of visible light-driven BiVO4: a review. J. Mater. Chem. A. 2017;5:16498–16521. doi: 10.1039/C7TA04441K. [DOI] [Google Scholar]
- 4.Luo W, et al. Solar hydrogen generation from seawater with a modified BiVO4 photoanode. Energy Environ. Sci. 2011;4:4046–4051. doi: 10.1039/c1ee01812d. [DOI] [Google Scholar]
- 5.Cho SK, Park HS, Lee HC, Nam KM, Bard AJ. Metal doping of BiVO4 by composite electrodeposition with improved photoelectrochemical water oxidation. J. Phys. Chem. C. 2013;117:23048–23056. doi: 10.1021/jp408619u. [DOI] [Google Scholar]
- 6.Zhou M, et al. Photoelectrodes based upon Mo: BiVO4 inverse opals for photoelectrochemical water splitting. Acs Nano. 2014;8:7088–7098. doi: 10.1021/nn501996a. [DOI] [PubMed] [Google Scholar]
- 7.Jo WJ, et al. Phosphate doping into monoclinic BiVO4 for enhanced photoelectrochemical water oxidation activity. Angew. Chem. Int. Ed. 2012;124:3201–3205. doi: 10.1002/ange.201108276. [DOI] [PubMed] [Google Scholar]
- 8.Zhou Y, et al. Highly efficient photoelectrochemical water splitting from hierarchical WO3/BiVO4 nanoporous sphere arrays. Nano lett. 2017;17:8012–8017. doi: 10.1021/acs.nanolett.7b04626. [DOI] [PubMed] [Google Scholar]
- 9.Han HS, et al. Boosting the solar water oxidation performance of a BiVO4 photoanode by crystallographic orientation control. Energy Environ. Sci. 2018;11:1299–1306. doi: 10.1039/C8EE00125A. [DOI] [Google Scholar]
- 10.Wang S, et al. New BiVO4 dual photoanodes with enriched oxygen vacancies for efficient solar-driven water splitting. Adv. Mater. 2018;30:1800486. doi: 10.1002/adma.201800486. [DOI] [PubMed] [Google Scholar]
- 11.Zhong DK, Choi S, Gamelin DR. Near-complete suppression of surface recombination in solar photoelectrolysis by “Co-Pi” catalyst-modified W:BiVO4. J. Am. Chem. Soc. 2011;133:18370–18377. doi: 10.1021/ja207348x. [DOI] [PubMed] [Google Scholar]
- 12.Zhang B, Wang L, Zhang Y, Ding Y, Bi Y. Ultrathin FeOOH nanolayers with abundant oxygen vacancies on BiVO4 photoanodes for efficient water oxidation. Angew. Chem. Int. Ed. 2018;57:2248–2252. doi: 10.1002/anie.201712499. [DOI] [PubMed] [Google Scholar]
- 13.Kim TW, Choi KS. Nanoporous BiVO4 photoanodes with dual-layer oxygen evolution catalysts for solar water splitting. Science. 2014;343:990–994. doi: 10.1126/science.1246913. [DOI] [PubMed] [Google Scholar]
- 14.Ye KH, et al. Carbon quantum dots as a visible light sensitizer to significantly increase the solar water splitting performance of bismuth vanadate photoanodes. Energy Environ. Sci. 2017;10:772–779. doi: 10.1039/C6EE03442J. [DOI] [Google Scholar]
- 15.Zhang K, Ma M, Li P, Wang DH, Park JH. Water splitting progress in tandem devices: moving photolysis beyond electrolysis. Adv. Energy Mater. 2016;6:1600602. doi: 10.1002/aenm.201600602. [DOI] [Google Scholar]
- 16.Abdi FF, et al. Efficient solar water splitting by enhanced charge separation in a bismuth vanadate-silicon tandem photoelectrode. Nat. Commun. 2013;4:2195. doi: 10.1038/ncomms3195. [DOI] [PubMed] [Google Scholar]
- 17.Zachäus C, Abdi FF, Peter LM, van de Krol R. Photocurrent of BiVO4 is limited by surface recombination, not surface catalysis. Chem. Sci. 2017;8:3712–3719. doi: 10.1039/C7SC00363C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pham TA, Ping Y, Galli G. Modelling heterogeneous interfaces for solar water splitting. Nat. Mater. 2017;16:401. doi: 10.1038/nmat4803. [DOI] [PubMed] [Google Scholar]
- 19.Trześniewski BJ, et al. Near-complete suppression of surface losses and total internal quantum efficiency in BiVO4 photoanodes. Energy Environ. Sci. 2017;10:1517–1529. doi: 10.1039/C6EE03677E. [DOI] [Google Scholar]
- 20.Li T, He J, Peña B, Berlinguette CP. Curing BiVO4 photoanodes with ultraviolet light enhances photoelectrocatalysis. Angew. Chem. Int. Ed. 2016;128:1801–1804. doi: 10.1002/ange.201509567. [DOI] [PubMed] [Google Scholar]
- 21.Zhong M, et al. Surface modification of CoOx loaded BiVO4 photoanodes with ultrathin p-Type NiO layers for improved solar water oxidation. J. Am. Chem. Soc. 2015;137:5053–5060. doi: 10.1021/jacs.5b00256. [DOI] [PubMed] [Google Scholar]
- 22.Chang X, et al. Enhanced surface reaction kinetics and charge separation of p-n heterojunction Co3O4/BiVO4 photoanodes. J. Am. Chem. Soc. 2015;137:8356–8359. doi: 10.1021/jacs.5b04186. [DOI] [PubMed] [Google Scholar]
- 23.Li L, et al. Black phosphorus field-effect transistors. Nat. Nanotech. 2014;9:372. doi: 10.1038/nnano.2014.35. [DOI] [PubMed] [Google Scholar]
- 24.Xia F, Wang H, Jia Y. Rediscovering black phosphorus as an anisotropic layered material for optoelectronics and electronics. Nat. Commun. 2014;5:4458. doi: 10.1038/ncomms5458. [DOI] [PubMed] [Google Scholar]
- 25.Liang L, et al. Electronic bandgap and edge reconstruction in phosphorene materials. Nano Lett. 2014;14:6400–6406. doi: 10.1021/nl502892t. [DOI] [PubMed] [Google Scholar]
- 26.Zhu M, et al. Metal-free photocatalyst for H2 evolution in visible to near-infrared region: black phosphorus/graphitic carbon nitride. J. Am. Chem. Soc. 2017;139:13234–13242. doi: 10.1021/jacs.7b08416. [DOI] [PubMed] [Google Scholar]
- 27.Zhu M, Sun Z, Fujitsuka M, Majima T. Z-Scheme photocatalytic water splitting on a 2D heterostructure of black phosphorus/bismuth vanadate using visible light. Angew. Chem. Int. Ed. 2018;57:2160–2164. doi: 10.1002/anie.201711357. [DOI] [PubMed] [Google Scholar]
- 28.Zhu M, Cai X, Fujitsuka M, Zhang J, Majima T. Au/La2Ti2O7 nanostructures sensitized with black phosphorus for plasmon-enhanced photocatalytic hydrogen production in visible and near‐infrared light. Angew. Chem. Int. Ed. 2017;56:2064–2068. doi: 10.1002/anie.201612315. [DOI] [PubMed] [Google Scholar]
- 29.Zhu X, et al. Black phosphorus revisited: a missing metal-free elemental photocatalyst for visible light hydrogen evolution. Adv. Mater. 2017;29:1605776. doi: 10.1002/adma.201605776. [DOI] [PubMed] [Google Scholar]
- 30.Ran J, Zhu B, Qiao S. Phosphorene co‐catalyst advancing highly efficient visible-light photocatalytic hydrogen production. Angew. Chem. Int. Ed. 2017;56:10373–10377. doi: 10.1002/anie.201703827. [DOI] [PubMed] [Google Scholar]
- 31.Batmunkh M, et al. Electrocatalytic activity of a 2D phosphorene-based heteroelectrocatalyst for photoelectrochemical cells. Angew. Chem. Int. Ed. 2018;57:2644–2647. doi: 10.1002/anie.201712280. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, et al. Black phosphorus quantum dots. Angew. Chem. Int. Ed. 2015;54:3653–3657. doi: 10.1002/anie.201409400. [DOI] [PubMed] [Google Scholar]
- 33.Huang Y, et al. Interaction of black phosphorus with oxygen and water. Chem. Mater. 2016;28:8330–8339. doi: 10.1021/acs.chemmater.6b03592. [DOI] [Google Scholar]
- 34.Kudo A, Omori K, Kato H. A novel aqueous process for preparation of crystal form-controlled and highly crystalline BiVO4 powder from layered vanadates at room temperature and its photocatalytic and photophysical properties. J. Am. Chem. Soc. 1999;121:11459–11467. doi: 10.1021/ja992541y. [DOI] [Google Scholar]
- 35.Cooper JK, et al. Electronic structure of monoclinic BiVO4. Chem. Mater. 2014;26:5365–5373. doi: 10.1021/cm5025074. [DOI] [Google Scholar]
- 36.Zhang J, et al. Interface energy band alignment at the all-transparent p-n heterojunction based on NiO and BaSnO3. Appl. Phys. Lett. 2018;112:171605. doi: 10.1063/1.5029422. [DOI] [Google Scholar]
- 37.Pendlebury SR, et al. Ultrafast charge carrier recombination and trapping in hematite photoanodes under applied bias. J. Am. Chem. Soc. 2014;136:9854. doi: 10.1021/ja504473e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Grigioni I, Stamplecoskie KG, Selli E, Kamat P. Dynamics of photogenerated charge carriers in WO3/BiVO4 heterojunction photoanodes. J. Phys. Chem. C. 2015;119:20792. doi: 10.1021/acs.jpcc.5b05128. [DOI] [Google Scholar]
- 39.Zaffran J, Toroker MC. Designing efficient doped NiOOH catalysts for water splitting with first principles calculations. ChemistrySelect. 2016;1:911–916. doi: 10.1002/slct.201600135. [DOI] [Google Scholar]
- 40.Dotan H, Sivula K, Grätzel M, Rothschild A, Warren SC. Probing the photoelectrochemical properties of hematite (α-Fe2O3) electrodes using hydrogen peroxide as a hole scavenger. Energy Environ. Sci. 2011;4:958–964. doi: 10.1039/C0EE00570C. [DOI] [Google Scholar]
- 41.Klahr B, Gimenez S, Fabregat-Santiago F, Hamann T, Bisquert J. Water oxidation at hematite photoelectrodes: the role of surface states. J. Am. Chem. Soc. 2012;134:4294–4302. doi: 10.1021/ja210755h. [DOI] [PubMed] [Google Scholar]
- 42.De Respinis M, et al. Solar water splitting combining a BiVO4 light absorber with a Ru-based molecular cocatalyst. J. Phys. Chem. C. 2015;119:7275–7281. doi: 10.1021/acs.jpcc.5b00287. [DOI] [Google Scholar]
- 43.Klahr B, Gimenez S, Fabregat-Santiago F, Bisquert J, Hamann T. Photoelectrochemical and impedance spectroscopic investigation of water oxidation with “Co–Pi”-coated hematite electrodes. J. Am. Chem. Soc. 2012;134:16693–16700. doi: 10.1021/ja306427f. [DOI] [PubMed] [Google Scholar]
- 44.Liu EY, Thorne JE, He Y, Wang D. Understanding photocharging effects on bismuth vanadate. ACS Appl. Mater. Interfaces. 2017;9:22083–22087. doi: 10.1021/acsami.7b06528. [DOI] [PubMed] [Google Scholar]
- 45.Liu G, et al. A tantalum nitride photoanode modified with a hole-storage layer for highly stable solar water splitting. Angew. Chem. Int. Ed. 2014;126:7423–7427. doi: 10.1002/ange.201404697. [DOI] [PubMed] [Google Scholar]
- 46.Le Formal F, Sivula K, Grätzel M. The transient photocurrent and photovoltage behavior of a hematite photoanode under working conditions and the influence of surface treatments. J. Phys. Chem. C. 2012;116:26707–26720. doi: 10.1021/jp308591k. [DOI] [Google Scholar]
- 47.Lee DK, Choi KS. Enhancing long-term photostability of BiVO4 photoanodes for solar water splitting by tuning electrolyte composition. Nat. Energy. 2018;3:53. doi: 10.1038/s41560-017-0057-0. [DOI] [Google Scholar]
- 48.Kuang Y, et al. Ultrastable low-bias water splitting photoanodes via photocorrosion inhibition and in situ catalyst regeneration. Nat. Energy. 2016;2:16191. doi: 10.1038/nenergy.2016.191. [DOI] [Google Scholar]
- 49.Huang J, et al. CoOOH nanosheets with high mass activity for water oxidation. Angew. Chem., Int. Ed. 2015;54:8722. doi: 10.1002/anie.201502836. [DOI] [PubMed] [Google Scholar]
- 50.Li R, et al. Spatial separation of photogenerated electrons and holes among {010} and {110} crystal facets of BiVO4. Nat. Commun. 2013;4:1432. doi: 10.1038/ncomms2401. [DOI] [PubMed] [Google Scholar]
- 51.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 52.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 53.Harl J, Schimka L, Kresse G. Assessing the quality of the ranphase approximation for lattice constants and atomization energies of solids. Phys. Rev. B. 2010;81:115126. doi: 10.1103/PhysRevB.81.115126. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary information files and from the corresponding author upon reasonable request.