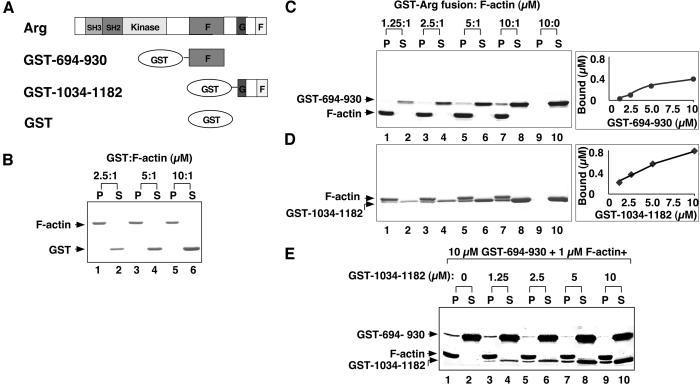
Figure 3.
Arg contains two independent F-actin binding domains. (A) Diagram of recombinant purified GST-Arg protein fragments. (B–D) Cosedimentation of GST or GST-Arg fragments with F-actin. A fixed concentration of F-actin (1 μM) was mixed with 2.5–10 μM GST (lanes 1–6) or 1.25–10 μM GST-Arg fragment fusion protein (lanes 1–8). For C and D, GST-fusion proteins (10 μM) were centrifuged also in the absence of F-actin as a control (lanes 9–10). After ultracentrifugation at 120,000 × g for 30 min, all the pellet fractions (P) and one third of the supernatant (S) fractions were subjected to SDS/PAGE followed by Coomassie blue staining. (B) GST; (C) GST-694–930; (D) GST-1034–1182. (E) Competitive binding of F-actin-binding domains to F-actin. For each reaction, F-actin (1 μM) was mixed with GST-694–930 (10 μM) and 0–10 μM GST-1034–1182. Samples were incubated, centrifuged, and analyzed as described for C and D.