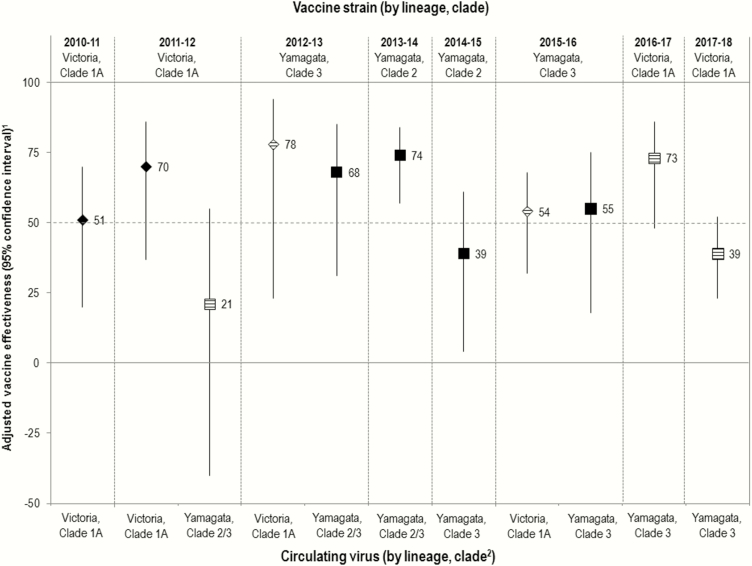
Figure 1.
Vaccine effectiveness (VE) against influenza B by season (n = 8) and scenario (n = 11) of lineage-level relatedness between the trivalent influenza vaccine (TIV) component and specified circulating viruses. VE estimates against Victoria-lineage viruses are shown as diamonds; VE estimates against Yamagata-lineage viruses are shown as squares. Where the TIV component and the specified circulating virus belong to different lineages, cross-lineage VE is represented as a hash mark. 1Sample sizes accompanying each estimate are provided in Supplementary Table 3. All analyses are adjusted for age group, sex, comorbidity, province, specimen collection interval, and week of specimen collection as detailed in Supplementary Table 4. Sensitivity analyses are presented in Supplementary Table 5. Differences in inclusion criteria, analysis period, and other model specifications should be taken into account when comparing to prior publications of season-specific VE estimates for influenza B (Supplementary References 24–29). 2See Supplementary Table 2 for details related to virological characterization. Prior to the 2013–2014 season, sequencing of the hemagglutinin gene was not routinely undertaken, and lineage and clade designation were based on alternative methods. In 2013–2014, most Yamagata viruses that were sequenced were clade 3 (82%) with some clade 2 contribution (18%). Yamagata clade 2 and clade 3 viruses can likely be considered antigenically distinct but with some variation in cross-reactivity based on antisera used (Supplementary References 18, 19).