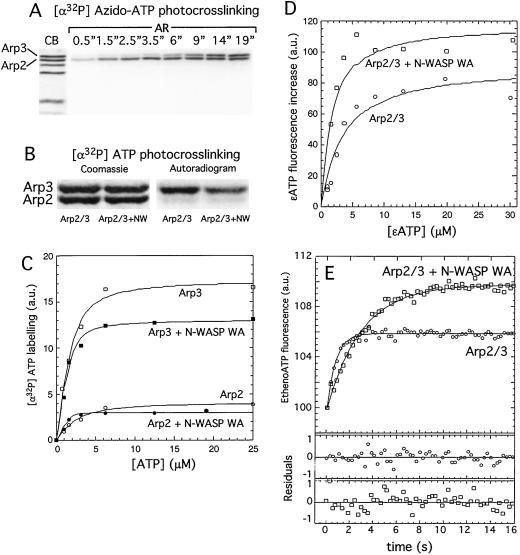
Figure 1.
Both the Arp2 and Arp3 subunits of the Arp2/3 complex bind nucleotide with micromolar affinity. (A) UV crosslinking of 5 μM [α-32P]azido-ATP to 1 μM Arp2/3 complex shows both Arp2 and Arp3 bind nucleotide and that [α-32P]azido-ATP crosslinking efficiency is greater on Arp2. (B) Crosslinking of ATP doped with [α-32P]ATP to 2 μM Arp2/3 complex by using UV light. In the presence of 100 μM ATP, radiolabeling occurs mainly on Arp3 with less efficient labeling on Arp2 both in the absence and presence of 25 μM N-WASP WA. (C) Quantitation of B for a range of ATP concentrations fit to the binding quadratic to obtain dissociation constants: Arp3 Kd = 0.6 μM; Arp2 Kd = 1.3 μM; Arp3 + N-WASP WA Kd = 0.25 μM; Arp2 + N-WASP WA Kd = 0.5 μM. Binding of N-WASP WA decreases the crosslinking efficiency. (D) Affinity of the Arp2/3 complex for etheno-ATP. Etheno-ATP fluorescence increase upon binding was measured for a range of etheno-ATP concentrations in the presence of a constant concentration of Arp2/3 complex by subtracting the contribution of free etheno-ATP fluorescence from the total measured intensity (see Materials and Methods). Arp2/3 complex alone binds etheno-ATP with an affinity of 3.1 μM, and the N-WASP–Arp2/3 complex binds etheno-ATP with an affinity of 1.3 μM. Binding of N-WASP WA enhances the etheno-ATP fluorescence signal. (E) ATP dissociation kinetics. In 5 μM ATP, nucleotide was driven from 1 μM Arp2/3 complex by mixing with 50 μM etheno-ATP, and binding kinetics were measured by fluorescence enhancement of etheno-ATP on binding. koff MgATP = 2.6 s−1. Dissociation slows in the presence of N-WASP WA koff MgATP = 0.7 s−1 and enhances the etheno-ATP fluorescence signal.