Figure 9.
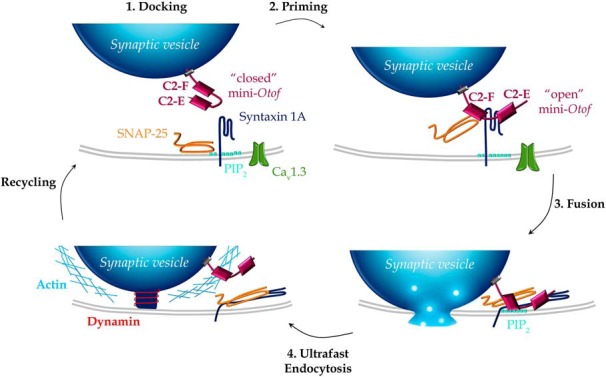
Schematic model for the engagement of the mini-otoferlin C2-EF in the synaptic vesicle cycle (exocytosis and endocytosis) at the IHC active zone. 1, Docking. The mini-Otof C2-EF, attached by its C-terminal transmembrane domain to the glutamatergic docked vesicles, is not yet engaged in direct interactions with the t-SNAREs (syntaxin 1A and SNAP-25) and the phospholipids PIP2 at the plasma membrane. At low intracellular Ca2+ concentration, when nearby Ca2+ channels are not voltage activated, the mini-otoferlin is folded, and its C2-E and C2-F domains interact with each other. 2, Priming. During voltage activation of the Ca2+ channels, [Ca2+]i increases and vesicles undergo a priming reaction where mini-Otof conformation shift from “closed” to “open” and interact with t-SNARE, forming a complex. Syntaxin 1A has been proposed to interact with C2-E and C2-F domains and SNAP-25 only with C2-F (Ramakrishnan et al., 2014). This step of the vesicle cycle is poorly restored by Otof-C2-EF, Otof-C2-DEF, Otof-C2-ACEF, and Otof-C2-ACDF (recruitment, Fig. 3G), explaining the absence of hearing restoration (Fig. 2). The molecular interactions of the mini-Otof complex with the Cav1.3 channels are not represented. 3, Fusion. When Ca2+ ions bind to the C2 domains of mini-Otof, the synaptic vesicle membrane and the plasma membrane are forced into close proximity by the SNARE complex, triggering partial insertion of the C2-F domain into PIP2 and resulting in vesicle fusion. This step of vesicle fusion is partially restored by Otof-C2-EF, Otof-C2-DEF, and Otof-C2-ACEF. 4, Ultrafast vesicle endocytosis. This is mediated by dynamin, which pinches off the vesicular membrane with the help of filamentous F-actin for initializing the membrane curvature. This ultrafast endocytosis was efficiently observed with Otof-C2-EF, Otof-C2-DEF, Otof-C2-ACEF, and Otof-C2-ACDF that could interact with the dynamin process.
