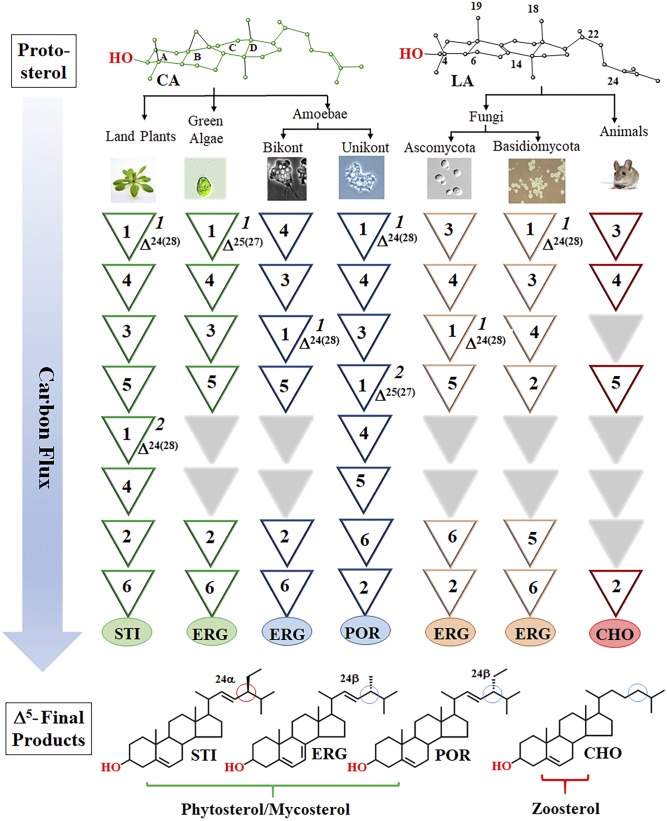
Fig. 1.
Variations in metabolite order in sterol biosynthesis. The numbers in inverted triangles indicate one or more enzymatic steps: step 1, sterol 24- or 28-methylation (1, 24-SMT; 2, 28-SMT); step 2, sterol 25(27)- or 24(25)-, or 24(28)-reduction; step 3, sterol C14-demethylation; step 4, - sterol C4-demethylation; step 5, several biosynthetic steps that cluster after C14-demethylation involve B/C-ring metabolism of sterol C8 to C7 isomerization, sterol C5-desaturation, or C7-reduction to give the typical sequence of Δ8 to Δ7 to Δ5,7 to Δ5; step 6, sterol C22-desaturation (compare supplemental Fig. S1A). The Δ5-sterol products: STI, stigmasterol; ERG, ergosterol; CHO, cholesterol. The six representative sterol biosynthesis pathways are based on chemical identifications, labeling studies, and kinetic properties of pure enzymes isolated from land plants, green algae, bikont and unikont amoebae, ascomycete and basidiomycete fungi, and vertebrate animals (2, 8–11).