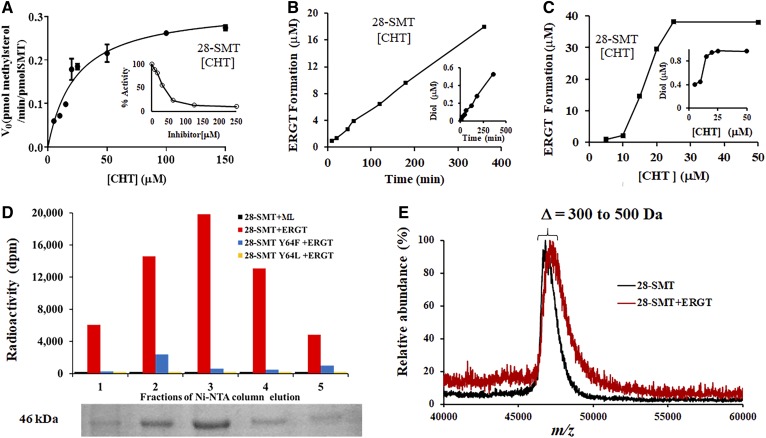
Fig. 6.
Kinetic, radiochemical, and proteomic analyses of yeast sterol analogs complexed with 28-AcSMT. A: Plot of the initial velocity of a SMT-catalyzed reaction versus the substrate concentration (inset is the IC50 of CHT against the Km of 24(28)-methylene lophenol substrate). B: Plot of time of incubation against CHT conversion to C28-steroidal monol and diol. C: Plot of increasing concentration of CHT against product formation of C28-steroidal monol and diol. To limit C29-sterol formation, the SAM concentration is fixed at 100 μM (supplemental Fig. S12). D: SDS-PAGE gel of different Ni-NTA column fractions containing 28-AcSMT complexed with ERGT versus radioactivity detected in each fraction (see the Materials and Methods for details of the binding experiment). F: Mass spectral analysis of ligand-free and ligand-bound (ERGT) 28-AcSMT.