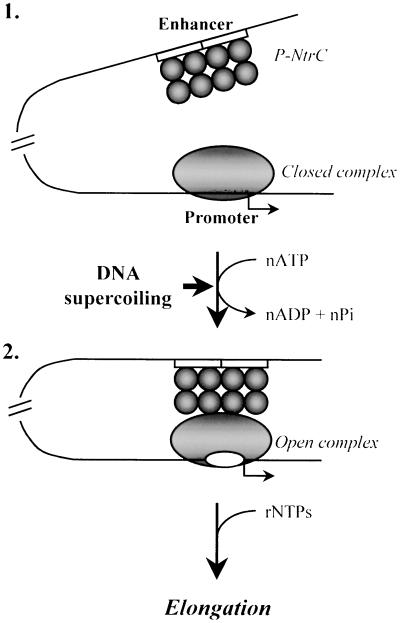
Figure 5.
Mechanism of activation of the glnAp2 promoter by the NtrC-dependent enhancer. (1) Before transcription of NtrC-dependent genes is induced, the Eσ54 holoenzyme forms a closed complex (RPc) at the promoter (localized at the −24 to −12 DNA region) but cannot initiate transcription. NtrC is bound to the enhancer (two 17-bp NtrC-binding sites are indicated by open boxes) but cannot communicate with the promoter. (2) After induction and phosphorylation by NtrB, NtrC forms higher-order homo-oligomers (not shown) and interacts with the holoenzyme, causing looping of the intervening DNA- and ATP-dependent formation of the open complex at the promoter. Enhancer–promoter communication over a distance is greatly facilitated by negative DNA supercoiling. After formation of the open complex is completed, enhancer–promoter interaction is broken and the DNA loop is opened (ref. 21; data not shown). As the RNA polymerase leaves the promoter, the σ54 subunit dissociates into solution (data not shown).