Abstract
Rapid eye movement (REM) sleep behavior disorder (RBD) involves REM sleep without atonia in conjunction with a recurrent nocturnal dream enactment behavior, with vocalizations such as shouting and screaming, and motor behaviors such as punching and kicking. Secondary RBD is well described in association with neurological disorders including Parkinson’s disease (PD), multiple system atrophy (MSA), and other conditions involving brainstem structures such as tumors. However, RBD alone is now considered to be a potential harbinger of later development of neurodegenerative disorders, in particular PD, MSA, dementia with Lewy bodies (DLB), and pure autonomic failure. These conditions are linked by their underpinning pathology of alpha-synuclein protein aggregation. In RBD, it is therefore important to recognize the potential risk for later development of an alpha-synucleinopathy, and to investigate for other potential causes such as medications. Other signs and symptoms have been described in RBD, such as orthostatic hypotension, or depression. While it is important to recognize these features to improve patient management, they may ultimately provide clinical clues that will lead to risk stratification for phenoconversion. A critical need is to improve our ability to counsel patients, particularly with regard to prognosis. The ability to identify who, of those with RBD, is at high risk for later neurodegenerative disorders will be paramount, and would in addition advance our understanding of the prodromal stages of the alpha-synucleinopathies. Moreover, recognition of at-risk individuals for neurodegenerative disorders may ultimately provide a platform for the testing of possible neuroprotective agents for these neurodegenerative disorders.
Keywords: REM sleep, REM behavior disorder, alpha-synucleinopathy
Introduction
Rapid eye movement (REM) sleep, which occupies approximately 20–25% of total sleep time, is a cyclical sleep state, occurring in intervals of 90–120 minutes during the night. Normally, during REM sleep, there is active inhibition of motor activity, which results in complete or near- complete muscle atonia. Atonia results from an interplay of multiple neurotransmitter systems, with a decrease in excitatory activity and increase in the inhibitory glycinergic and GABAergic premotor neuronal input to motor neurons.
However, the processes resulting in REM atonia can become disrupted, leading to REM sleep without atonia (RSWA)(Barone et al. 2015), which is a finding noted during an overnight polysomnogram (PSG) via abnormally increased electromyogram tone during REM sleep. REM sleep behavior disorder (RBD) is an abnormal condition consisting of RSWA in conjunction with a history of recurrent nocturnal dream enactment behavior.
The diagnosis of idiopathic RBD occurs when none of the conditions known to cause secondary RBD are present (see below), and when other conditions with possible abnormal nocturnal behaviors have been ruled out (obstructive sleep apnea or epilepsy, for example)(Iranzo and Santamaria 2005, Peever et al. 2014). Potential risk factors for RBD include smoking, head injury, pesticide exposure, and having worked as a farmer(Postuma et al. 2012).
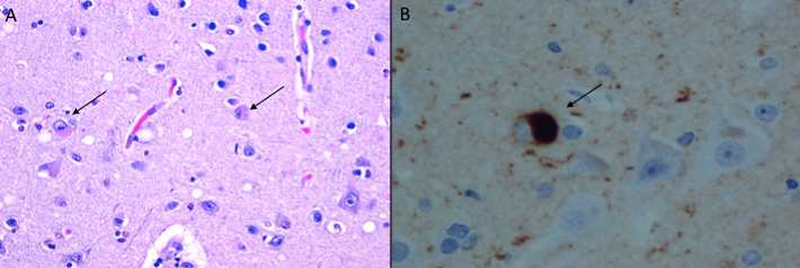
The diagnosis of secondary RBD occurs when there is an associated condition preceding it and likely contributing to its etiology(Peever et al. 2014), the most important being the alpha-synucleinopathies (Table 1). These conditions, including Parkinson’s disease (PD), multiple system atrophy (MSA), dementia with Lewy bodies (DLB)(Gagnon et al. 2006, Manni et al. 2011), and pure autonomic failure (PAF)(Kaufmann et al. 2017) are so named because of the characteristic intracellular protein accumulation of alpha-synuclein, that may be visualized in a subset of synucleinopathies (for example PD and DLB) as Lewy bodies (Figure 1)(Postuma et al. 2009, Classen and Schnitzler 2010). It is unclear whether RBD is linked to the alpha-synucleinopathies via aggregated intracellular material, or through a common anatomic pathology(Ebben et al. 2012).
Table 1:
Major clinical and pathological features of the alpha-synucleinopathies
| Neurological disorder | Major clinical manifestations | Pathology |
|---|---|---|
| Parkinson’s disease | Bradykinesia, muscle rigidity, rest tremor, postural instability | Alpha-synuclein-containing intracytoplasmic Lewy bodies in neurons and Lewy neurites |
| Dementia with Lewy bodies | Dementia, fluctuating clinical status, hallucinations, parkinsonism | Alpha-synuclein-containing intracytoplasmic Lewy bodies in neurons and Lewy neurites |
| Multiple system atrophy | Autonomic dysfunction, parkinsonism, cerebellar signs in some | Alpha-synuclein containing glial cytoplasmic inclusions |
| Pure autonomic failure | Autonomic dysfunction | Alpha-synuclein-containing intracytoplasmic Lewy bodies in neurons and Lewy neurites |
Figure 1:
Lewy bodies in the cortex of an individual with dementia with Lewy bodies (DLB) visualized by (A) hematoxylin and eosin staining, and (B) high magnification of immunostaining for alpha-synuclein, demonstrating intracytoplasmic localization of the Lewy body. Arrows mark examples of Lewy bodies. Photomicrographs courtesy of Dr Ehud Lavi, Weill Cornell Medical Center).
Of import is the fact that, in addition to secondary RBD occurring in the alpha-synucleinopathies, RBD can also precede the onset of alpha-synucleinopathy by decades(Iranzo et al. 2014). RBD is now therefore described as part of the “pre-motor” PD stage, and many individuals with RBD will develop PD(Iranzo et al. 2014, Visanji and Marras 2015). As a result, an underlying pathology that impacts both REM atonia and midbrain dopaminergic centers has been postulated(Chen et al. 2013). This idea aligns with earlier publications postulating a spread of alpha-synuclein pathology through brainstem and other regions that could explain sleep abnormalities preceding motor abnormalities on an anatomical basis, the “Braak hypothesis”(Braak et al. 2003). Research into RBD therefore would ideally improve our ability to counsel patients, particularly with regard to prognosis, and would advance our understanding of the prodromal stages of the alpha-synucleinopathies. Moreover, recognition of at-risk individuals for neurodegenerative disorders may ultimately provide a platform for the testing of possible neuroprotective agents for these neurodegenerative disorders.
In this review, we address the underlying physiology of REM sleep, the putative pathophysiology of RSWA and RBD, the conditions known to be associated with RBD, including the alpha-synucleopathies, and the clinical factors that seem to herald the emergence of neurodegeneration in the context of RBD(Iranzo et al. 2016).
Rapid Eye Movement Sleep and Muscle Control
REM sleep, first described in 1953(Aserinsky and Kleitman 1953), consists of low voltage fast electroencephalographic (EEG) activity, rapid eye movements, and muscle atonia. REM sleep is informally known as “dream sleep” due to the finding that waking subjects from REM sleep results in reports of dreaming(Dement and Kleitman 1957). Initial experiments(Aserinsky and Kleitman 1953, Dement and Kleitman 1957, Jouvet 1962) demonstrated the characteristic features of EEG and electromyogram (EMG) parameters that have come to define REM sleep. Normal REM sleep has been demonstrated to include corticohippocampal activation similar to wakefulness in addition to atonia of the postural muscles to prevent movement(Chen et al. 2013).
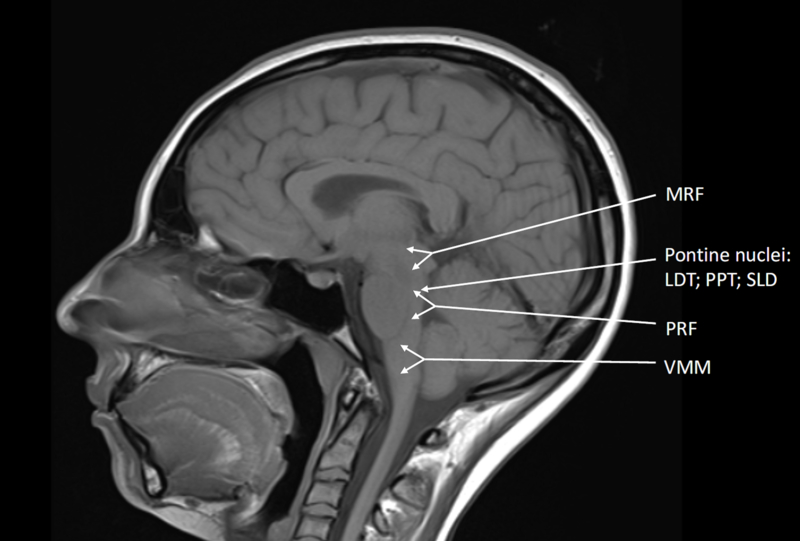
Evidence supports a number of key structures within the brainstem, whose activity is precisely integrated to produce REM sleep (Figure 2). Proposed models of REM sleep physiology(Ng and Pavlova 2013) involve the laterodorsal tegmentum (LDT) and the pedunculopontine tegmentum (PPT), which reside in the pontomesencephalic junction of the brainstem and consist of two populations of cholinergic neurons(Mitani et al. 1988). There exist a group of neurons most active in REM sleep, and another group active in both REM sleep and wakefulness(Luebke et al. 1992, Leonard and Llinas 1994, McCarley et al. 1995). Via muscarinic and nicotinic acetylcholine receptors, connections from the LDT and PPT send excitatory signals to neurons in the pontine reticular formation (PRF) and mesencephalic reticular formation (MRF), which then result in producing the classic characteristics of REM sleep such as low voltage fast EEG activity, rapid eye movements, and muscle atonia(Ng and Pavlova 2013).
Figure 2:
A number of key structures within the brainstem are integrated into a network to produce REM sleep, and alterations in their activities may occur in RBD. Cholinergic signals from the LDT and PPT activating the PRF and MRF lead to cardinal features of REM sleep. The SLD contains neurons that are highly active in REM sleep, and contribute to muscle atonia via signals to the spinal cord (layer VII) and the VMM. MRF: mesencephalic reticular formation; LDT: laterodorsal tegmentum; PPT: pedunculopontine tegmentum; PRF: pontine reticular formation; SLD: sublaterodorsal tegmental nucleus; VMM: Ventromedial medulla
Muscle control in REM sleep is mostly based in the pons(Baghdoyan et al. 1984, Boissard et al. 2002, Lu et al. 2006), and has been reported under different names, including the pontine inhibitory area, subcoeruleus nucleus, nucleus reticularis pontis oralis (dorsal division), perilocus coeruleus alpha, and the sublaterodorsal tegmental nucleus (SLD)(Peever et al. 2014), the latter being the term used in this review.
The neurons of the SLD, most active during REM sleep(Siegel et al. 1991, Maloney et al. 1999, Boissard et al. 2002, Boissard et al. 2003, Lu et al. 2006), release glutamate(Clement et al. 2011) to trigger muscle atonia(Lai and Siegel 1988, Boissard et al. 2002). Experimentally produced small lesions of the SLD result in RSWA without affecting REM sleep amount, whereas larger lesions not only produce RSWA, but also affect the amount and duration of REM sleep(Boissard et al. 2002, Lu et al. 2006). Thus, as REM sleep architecture is generally unaffected in RBD, it is likely that there exists a smaller lesion of SLD neurons in this condition(Peever et al. 2014). SLD neurons send glutamatergic signals to the inhibitory glycinergic/GABAergic interneurons in layer VII of the spinal cord(Vetrivelan et al. 2009), and it has been proposed that these interneurons in turn antagonize excitatory inputs from the motor cortex and brainstem to motor neurons(Lu et al. 2006, Chen et al. 2013). REM atonia may also be achieved by SLD neurons activating inhibitory circuits located in the ventromedial medulla (VMM)(Lai and Siegel 1988, Schenkel and Siegel 1989, Holmes et al. 1994). Neurons of the VMM are hypothesized to send glycinergic projections directly to spinal motor neurons(Hossaini et al. 2012) and activate GABA-and glycine-containing neurons in the ventral and alpha gigantocellular reticular nucleus, which would then inhibit skeletal motor neurons(Lai and Siegel 1988, Vetrivelan et al. 2009, Peever et al. 2014).
Studies suggest that excessive phasic movements in RBD may result, at least in part, from lack of normal input from GABA and glycine sources(Peever et al. 2014). Intracellular recording studies demonstrate that bursts of motor neuron excitation, resulting from glutamatergic inputs, leads to muscle twitches during REM sleep(Soja et al. 1995); blocking glutamate receptors prevents these twitches(Burgess et al. 2008). Additionally, GABA and glycine inputs normally inhibit REM muscle twitches(Brooks and Peever 2008), and pharmacological blockade of these inputs increases glutamate-induced motor twitches during REM sleep(Brooks and Peever 2008, Brooks and Peever 2012).
Since motor neurons receive both inhibitory and excitatory inputs during the phasic periods of REM sleep, the stronger input determines how they respond. In fact, during phasic periods, the postsynaptic inhibitory drives that control motor neuron activity during the tonic periods are actually enhanced(Chase and Morales 1982, Morales and Chase 1982, Chase and Morales 1983), but when excitatory inputs are more potent, rapid eye movements, and twitches/jerks of postural, masseter, digastric, hypoglossal, ocular, diaphragmatic, lingual and other muscles occurs(Chase 2013).
REM Sleep Behavior Disorder
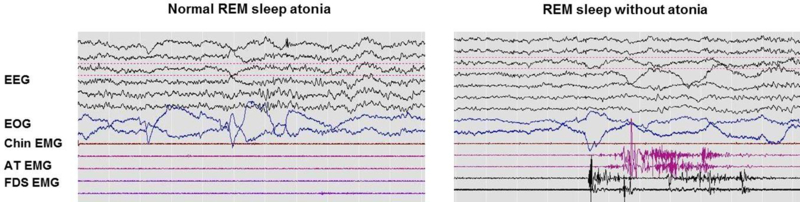
RBD is a parasomnia consisting of RSWA in combination with a history of recurrent nocturnal dream enactment behavior(Schenck et al. 1986, Olson et al. 2000, Schenck and Mahowald 2002, Arnulf 2012). According to the International Classification of Sleep Disorders, 3rd edition (ICSD-3), the clinical diagnosis of RBD requires: 1) the presence of RSWA on overnight PSG and 2) either sleep-related injurious, potentially injurious, or disruptive behaviors by history, and/or abnormal REM sleep behavior documented during PSG monitoring(American Academy of Sleep Medicine 2014 ). Additionally, there must be an absence of epileptiform activity on electroencephalography during REM sleep, and the sleep disorder cannot be better explained by another sleep disorder, medical or neurological disorder, mental disorder, medication use, or substance use disorder (Figure 3).
Figure 3:
A comparison between normal REM sleep and elevated muscle tone during REM sleep, taken from polysomnographic records. EEG: electroencephalography; EOG: electrooculogram; EMG: electromyogram; AT: anterior tibialis; FDS: flexor digitorum superficialis
While the neural circuitry involved in RBD has not yet been identified, researchers have proposed various regions, including the primary and premotor cortices, with input from the basal ganglia, or from brainstem or spinal cord motor generators(Grillner and Zangger 1979, Noga et al. 1988, Blumberg and Lucas 1994). Resultant muscle activity ranges from twitches to dream mentation-linked actions(Manni et al. 2009), possibly associated with violent, defensive nightmares(Olson et al. 2000).
The overall prevalence of RBD is not known, but has been estimated to be 0.4–0.5% in the general population(Ohayon et al. 1997), whereas up to 2% may be affected after the age of 60(Kang et al. 2013) and up to 6%, after the age of 70(Boot et al. 2012). While RBD is found predominantly in men, there may an under-diagnosis in women due to less violent REM sleep behaviors(Peever et al. 2014).
It is unknown if there is a truly genetic basis for RBD. However, a questionnaire-based study revealed a positive family history of dream enactment behaviors for RBD (14%) compared with controls (5%)(Dauvilliers et al. 2013). Additionally, an association has been reported between RBD and human leukocyte antigen (HLA) class II genes, one study demonstrating the presence of the DQwl (DQB1*05, 06) allele in 84% of Caucasian men with RDB were positive compared with 56% in the control group(Schenck et al. 1996).
Conditions Associated with RBD
It has been reported that roughly half of PD patients have RSWA or RBD(Gagnon et al. 2002, Iranzo et al. 2006, Sixel-Doring et al. 2011), and PD patients with comorbid RBD may present with more rapid motor progression and cognitive dysfunction. A recent study found alterations in the left and right cingulum and left and left inferior occipital fasciculus in PD patients with RBD, suggesting that these areas may serve as potential landmarks in understanding the brain changes that occur with PD(Rahmani et al. 2016). No difference in RBD prevalence was found between men and women with PD in another recent study, and women were noted to report significantly fewer aggressive behaviors during REM sleep(Bjornara et al. 2013). The presence of RBD is also considered to be supportive of the diagnosis of DLB(Boeve et al. 2007). Ferman et al followed 234 consecutive patients with dementia until autopsy, and patients with a history of RBD were 6 times more likely to have autopsy-confirmed DLB than other neurodegenerative dementia conditions(Ferman et al. 2011). Similarly, most patients with MSA exhibit RBD(Iranzo et al. 2005). One study of 42 MSA patients demonstrated that RBD is present in up to 88%, and was even present in some patients that reported no symptoms; in this cross-sectional analysis, more than half of MSA patients report symptoms of RBD before the onset of motor deficits(Palma et al. 2015).
Despite the recent focus on alpha-synucleinopthies, multiple other conditions have been reported in association with RBD (Table 2). Other degenerative diseases associated with RBD, such as Huntington’s disease, Alzheimer’s disease, corticobasal degeneration(Lo Coco et al. 2009), and amyotrophic lateral sclerosis(Ebben et al. 2012) have been reported. RSWA and RBD have also been reported in acute inflammatory demyelinating polyneuropathy (Guillain–Barré syndrome), and in several of these cases spontaneous complete remission occurred concomitantly with neurological recovery(Schenck et al. 1986, Cochen et al. 2005).
Table 2:
Neurological disorders associated with RBD in adults
| Neurological disorder | Nature of association | Reference |
|---|---|---|
| Parkinson’s disease | Approximately 50% with PD have RBD May be associated with more rapid motor progression + cognitive dysfunction Bilateral cingulum and inferior occipital fasciculus alterations |
(Iranzo, Molinuevo et al. 2006) (Gagnon 2002) (Sixel-Doring 2011) |
| Dementia with Lewy bodies | Cognitive deficits reported in RBD are seen mostly in patients with dementia risk Cognitive tests assessing attention and executive functions are useful in early detection of DLB in RBD patients |
(Ferman TJ 2011) (Boeve, Silber et al. 1998) |
| Multiple system atrophy | Present in the majority of cases | (Iranzo 2005) |
| Pure autonomic failure | Possible association with risk of developing MSA | (Plazzi, Cortelli et al. 1998) |
| Progressive supranuclear palsy | Reported in a minority of cases | (De Cock, Lannuzel et al. 2007) (Munhoz and Teive 2014) |
| Guadeloupian atypical parkinsonism | Present in the majority of indivuduals (single small study) | (De Cock, Lannuzel et al. 2007) |
| Huntington’s disease | Anecdotally reported: conflicting reports exist | (Lo Coco 2009) |
| Amyotrophic lateral sclerosis | Anecdotally reported: case study describing two siblings with familial ALS | (Ebben 2012) (Lo Coco 2009) |
| Alzheimer’s disease | Anecdotally reported: rare RSWA may be more commonly seen |
(Lo Coco 2009) |
| Guillain-Barre syndrome | Anecdotally reported: in some cases resolved with neurological recovery | (Schenck 1986) (Cochen 2005) |
| Stroke | Anecdotally reported: reported in brainstem infarcts | (Culebras 1989) (Kimura 2000) (Xi 2009) (Provini 2004) |
| Tumor | Anecdotally reported: reported in brainstem tumors | (Zambelis 2002) (Schenck 2002) |
| Demyelinating disorders | Anecdotally reported: seen in context of brainstem lesions RBD can be the first sign of multiple sclerosis |
(Plazzi 2002) (Tippmann-Peikert 2006) (Gomez-Choco 2007) |
| Inflammatory lesions | Anecdotally reported: single case report Small MRI hypointensities found in pontine tegmentum and dorsal medulla, suggesting post-inflammatory lesions that persisted between acute episodes |
(Limousin 2009) |
| Limbic encephalitis | Anecdotally reported. Data from animal studies suggest the limbic system may be involved in the pathogenesis of RBD, particularly the amygdala. | (Manni 2011) (Compta 2007) (Lin 2009) |
| PTSD | Anecdotally reported. Hypothesized that increased noradrenaline turnover can result from repeated traumas, which then may result in its depletion in locus coeruleus, which may, in turn, inhibit cholinergic laterodorsal tegmentum nucleus | (Husain 2001) |
| Narcolepsy with cataplexy | 50% exhibit RSWA RBD in narcolepsy tends to occur at a much younger age than in idiopathic RBD |
(Olson 2000) (Ferri 2008) (Mattarozzi 2008) (Nightingale 2005) (Schenck 1992) (Dauvilliers 2007) |
RBD has been demonstrated following focal neurological insults at the brainstem level, including those from ischemic(Culebras and Moore 1989, Kimura et al. 2000, Xi and Luning 2009) or hemorrhagic(Provini et al. 2004) strokes, brainstem tumors(Schenck and Mahowald 2002, Zambelis et al. 2002), demyelinating plaques(Plazzi and Montagna 2002, Tippmann-Peikert et al. 2006, Gomez-Choco et al. 2007), and inflammatory lesions(Limousin et al. 2009). In all of the observations listed, an area of the pons was damaged; for example, focal lesions of the SLD due to stroke or inflammation may produce RBD(Iranzo and Aparicio 2009); and animal studies have demonstrated that unilateral lesions are sufficient(Zagrodzka et al. 1998, Boeve et al. 2007).
One case of acute RBD occurred immediately following implantation of an electrode into the subthalamic nucleus as a treatment for PD. It was hypothesized that a microlesion in or near the pars compacta of the substantia nigra was the direct cause of RBD, via the interruption of descending inputs to the pontine REM sleep regulatory regions. The authors also surmised that a substantia nigra lesion might have been responsible for the emergence of RBD, suggesting that basal ganglia circuits could potentially be involved in motor control during REM sleep(Piette et al. 2007).
Limbic encephalitis has been demonstrated as having an association with RBD(Iranzo et al. 2006, Compta et al. 2007, Lin et al. 2009, Manni et al. 2011), but as there has been a lack of detected brainstem involvement in the cases reported, the hypothesis of an involvement of the limbic system in the pathogenesis of RBD(Manni et al. 2011) has been raised. The limbic system is activated during normal REM sleep, resulting in the emotional content of dreams(Hobson and Pace-Schott 2002), and data from animal studies imply that the limbic system may play a role in the pathogenesis of RBD, especially the amygdala(Zagrodzka et al. 1998, Boeve et al. 2007).
There is one case with PSG assessment in the context of alcohol withdrawal reported in the literature, which demonstrated disruption of the sleep-wake cycle, and a state similar to RSWA directly arising from wakefulness. Chronic alcohol abuse is known to down-regulate GABA-ergic post-synaptic receptors, and the authors hypothesized that acute alcohol withdrawal led to impairment of limbic system GABA-ergic transmission(Plazzi et al. 2002).
There exist reports of RBD occurring in the context of mental stressors. For example, in a study of 27 military veterans with RBD, 15 had post-traumatic stress disorder (PTSD), and 20 were identified who reported a previous major stressful event(Husain et al. 2001). One hypothesis for this association is that increased noradrenaline turnover can result from repeated traumas, which then may result in its depletion in the LC, which may, in turn, inhibit the cholinergic LDT nucleus. Hence, LC dysfunction may play a role in both RBD and PTSD(Husain et al. 2001) as the LC and LDT nuclei are involved in REM sleep regulation(Manni et al. 2011).
RSWA and/or RBD are common in narcolepsy with cataplexy, but rare in narcolepsy without cataplexy(Schenck and Mahowald 1992, Olson et al. 2000, Nightingale et al. 2005, Ferri et al. 2008, Mattarozzi et al. 2008); one study of 16 patients with narcolepsy and cataplexy found that 50% exhibit RSWA(Dauvilliers et al. 2007). RBD in the context of narcolepsy tends to occur at a much younger age than RBD (may even appear during childhood(Bonakis et al. 2009, Lloyd et al. 2012)), and it has been hypothesized that narcoleptic patients with RBD display a distinct form of the disease(Oudiette et al. 2012). While hypocretin deficiency is a suggested reason for RSWA in narcolepsy with cataplexy, the mechanism in those without cataplexy is not known(Knudsen et al. 2010, Khalil et al. 2013). Interestingly, a recent study measuring six cerebrospinal fluid biomarkers of neurodegeneration, including alpha-synuclein and beta-amyloid, found no evidence to suggest tauopathy, synucleinopathy, and immunopathy in narcolepsy patients(Jennum et al. 2016). Given this data, how and why exactly RSWA and RBD are common in cases of narcolepsy with cataplexy is not known.
Finally, it is of import to note that children may develop RBD, which is typically associated with neurodevelopmental disabilities, narcolepsy, or the use of certain medications. It is believed that the etiology of childhood RBD is distinct from RBD in adults, as none have been reported to suffer with an extrapyramidal neurodegenerative disorder(Lloyd et al. 2012).
Medications Precipitating RSWA and RBD
Selective serotonin reuptake inhibitors (SSRIs) and selective norepinephrine reuptake inhibitors (SNRIs) have well-known associations with RSWA and RBD(Lee et al. 2016). One study found that 15% of patients taking the SSRI fluoxetine in a sleep clinic population were shown to have RSWA(Schenck et al. 1992), and other studies have demonstrated an increased prevalence of RSWA in patients taking antidepressants(Hoque and Chesson 2010, Boeve et al. 2013, Zhang et al. 2013). Recently, one study demonstrated a nearly 10-fold increase in risk for RSWA while taking these medications(Lee et al. 2016).
It is estimated that the odds ratio of developing RBD is 1.9 with antidepressant use(Hoque and Chesson 2010), but the factors that predict the risk of developing RSWA while on SSRI/SNRIs and its prevalence remains unknown. Other medications with known associations to RSWA and RBD include the tricyclic antidepressants (amitriptyline, nortriptyline, imipramine, clomipramine, desipramine, protriptyline, trimipramine, and mirtazapine)(Hoque and Chesson 2010, Manni et al. 2011), and case reports have demonstrated acute RBD with the use of monoamine oxidase inhibitors (phenelzine and selegiline), a beta-adrenergic blocker (bisoprolol), and a cholinesterase inhibitor (rivastigmine)(Yeh et al. 2010).
It is thought that because these medications stimulate the serotonin system, and/or block acetylcholine transmission, they may induce RBD and RWSA(Winkelman and James 2004), possibly through prevention of normal sleep-related hypotonia (serotoninergic medications) or normal REM sleep-related atonia (anticholinergic medications). Additionally, the serotonergic medications may mediate REM sleep suppressive properties(Lu et al. 2006, Boeve et al. 2007), and produce a reversible alteration in the REM-on/REM-off circuitry, potentially leading to altered motor control during REM sleep(Manni et al. 2011).
At present, it is unclear whether antidepressant-associated RBD is a benign side effect of the medication, or, in fact, a marker of prodromal neurodegenerative disease. Postuma et al.(Postuma et al. 2013) reported that RBD patients taking antidepressants had less chance of developing neurodegenerative disease than those without antidepressant use. Similarly, a more recent study demonstrated that the prevalence of RBD among those on SSRI/SNRIs was lower than the larger group of sleep patients, which, as the authors point out, may be artifactual, or, it may represent the intriguing possibility that antidepressant exposure may be protective against RBD(Lee et al. 2016). However, the presence of comorbid depression in RBD further complicates interpretation of antidepressant association with RBD.
Although a few case reports have shown improvement of RSWA and RBD upon discontinuation of fluoxetine(Parish 2007, Applebee et al. 2009), there is a suggestion from other studies that antidepressants may “unmask” RBD rather than cause it(Postuma et al. 2013), and it has been demonstrated that RBD can persist for at least 19 months after discontinuation of SSRI(Winkelman and James 2004). Finally, markers of prodromal neurodegeneration may still be present in those with antidepressant-associated RBD, which suggests that antidepressants trigger early clinical presentation of an RBD that is nonetheless still due to underlying neurodegeneration(Jiang et al. 2016).
Other Signs and Symptoms in RBD
Clinical and subclinical symptoms in patients with RBD can be quite disparate, suggesting that the extent of the neurological process, whether neurodegenerative or other, differs greatly among those affected(Postuma et al. 2006, Postuma et al. 2009). Areas affected in RBD are not restricted to the brainstem regions regulating REM sleep(Fantini et al. 2006, Postuma et al. 2006, Postuma et al. 2009, Iranzo et al. 2010, Iranzo et al. 2011, Postuma et al. 2012, Iranzo et al. 2013, Frauscher et al. 2014, Aguirre-Mardones et al. 2015, Vilas et al. 2015, Ferini-Strambi et al. 2004, Miyamoto et al. 2006, Postuma et al. 2011, Scherfler et al. 2011, Terzaghi et al. 2013, Iranzo et al. 2014, Sprenger et al. 2015) but may extend to regions such as the olfactory system, the nigrostriatal system, and the autonomic system(Iranzo et al. 2016). Clinical examples of more diffuse effects that have been detected in those with RBD include subtle parkinsonian signs(Postuma et al. 2006, Postuma et al. 2009, Postuma et al. 2012), olfactory loss(Fantini et al. 2006, Postuma et al. 2011), depression(Frauscher et al. 2014, Vilas et al. 2015), autonomic dysfunction such as constipation or orthostatic hypotension(Postuma et al. 2006, Postuma et al. 2009), color vision impairment(Postuma et al. 2011), and subtle cognitive dysfunction (demonstrated only through cognitive tests)(Ferini-Strambi et al. 2004, Terzaghi et al. 2013, Youn et al. 2016) (Table 3).
Table 3.
Signs and symptoms associated with RBD
| Signs and symptoms | Description | Reference |
|---|---|---|
| Reduced heart rate variability | Index of beat to beat variability (RR standard deviation): 24.6 ± 2.2 msec in RBD vs 35.2 ± 3.5 msec in controls (p = 0.006) | (Postuma 2010) |
| Orthostatic hypotension | Systolic blood pressure drop 15.2 ± 2.1 mm Hg in RBD vs 3.7 ± 2.5 in controls (p = 0.003) | (Postuma, Gagnon et al. 2009) |
| Small fiber neuropathy | 39% in RBD vs 4.5% in controls | (Schrempf, Katona et al. 2016) |
| Hyposmia | 61.1% in RBD vs 16.6% in controls | (Fantini, Postuma et al. 2006) (Postuma, Gagnon et al. 2011) |
| Constipation | 0.13 ± 0.092 constipation symptoms in controls vs 0.73 ± 0.11 in RBD (p < 0.01) | (Postuma, Gagnon et al. 2009) |
| Depression | 28.8% in RBD vs 17.5% in controls (control subjects in this study were healthy and those with other sleep disorders) | (Frauscher, Jennum et al. 2014) (Vilas, Iranzo et al. 2015) |
| Cognitive dysfunction | 43.7% impaired in visuospatial constructional tests in RBD; 82.3% impaired in visuospatial learning test in RBD MCI in 35% in RBD |
(Ferini-Strambi, Di Gioia et al. 2004) (Terzaghi, Zucchella et al. 2013) (Youn, Kim et al. 2016) |
| Color vision impairment | Greater at baseline in individuals with RBD who subsequently developed neurodegenerative disease vs those who did not: adjusted Farnsworth Munsell −100 scores 153.0 ± 83.2 vs 129.1 ± 62 (p = 0.22) | (Postuma, Gagnon et al. 2011) |
| Subtle parkinsonian signs | Mean UPDRS score 6.0 ± 1.0 in RBD vs 3.0 ± 0.16 in controls (p = 0.023); 7/25 with RBD had detectable bradykinesia UPDRS part III (motor) score 5.8 ± 0.64 in RBD vs 2.70 ± 0.5 in controls (p < 0.001) |
(Postuma, Lang et al. 2006) (Postuma, Gagnon et al. 2009) (Postuma, Lang et al. 2012) |
Imaging also supports more widespread involvement in some with RBD, with reduced striatal dopamine uptake in dopamine transporter imaging(Iranzo et al. 2010, Iranzo et al. 2011), substantia nigra hyperechogenicity in midbrain transcranial sonography(Iranzo et al. 2014), reduced metaiodobenzylguanadine uptake in cardiac scintigraphy(Miyamoto et al. 2006), and increased diffusivity and decreased fractional anisotropy in the brainstem nuclei in diffusion tensor imaging(Scherfler et al. 2011).
Pathology in RBD is not limited to the central nervous system. Although pain and sensory symptoms are uncommon in RBD, decreased small nerve fiber density has been reported in epidermal biopsies(Schrempf et al. 2016). Moreoever, aggregates of phosphorylated alpha-synuclein characteristic of the alpha-synucleinopathies have been detected in some instances of RBD in biopsies of autonomic nerve fibers innervating organs such as the colon(Sprenger et al. 2015) and the submandibular gland(Iranzo et al. 2016, Vilas et al. 2016). Autonomic dysfunction is described in more detail in the following section since it has been particularly well studied.
Autonomic Dysfunction in RSWA and RBD
While the pathophysiological mechanisms of RBD are not fully understood(Gagnon et al. 2006), it is possible that brainstem structures contributing to the central autonomic network are affected(Lanfranchi et al. 2007). Those with RBD have been shown to have a reduction in heart rate variability (HRV), regardless of whether the development of alpha-synucleinopathy occurs in the future(Postuma et al. 2010). Interestingly, isolated RSWA was found to be associated with a similar dysfunction, although the clinical implications of this remain to be seen(Barone et al. 2015). While isolated RSWA is a clinical conundrum, in that its relevance is unclear presently(Boeve 2010), it has been postulated that autonomic dysfunction may be an integral component of the pathogenesis of RBD(Postuma et al. 2010), thus suggesting that isolated RSWA represents a preclinical form of RBD(Lam et al. 2013, Barone et al. 2015).
Additionally, through the use of cardiac metaiodobenzylguanidine scintigraphy, postganglionic degeneration of cardiac sympathetic neurons has been demonstrated in those with RBD(Miyamoto et al. 2006), as has the presence of abnormal beat-to-beat variability(Ferini-Strambi et al. 1996), an absence of REM-related cardiac and respiratory responses(Lanfranchi et al. 2007) and abnormalities in quantitative sudomotor axon reflex testing (QSART)(Benarroch et al. 2009). The majority of studies have demonstrated that in IRBD, autonomic dysfunction is present and predominantly involves the sympathetic and cardiovagal branches, but whether these dysfunctions in isolated RBD predict the development of a neurodegenerative disease is still controversial(Chiaro et al. 2017).
Despite only limited literature on the prevalence of RBD in PAF patients, intriguing possibilities have been suggested. For example, a recent prospective cohort of patients with PAF by Kaufmann showed that only those with RBD phenoconverted to PD, MSA, and DLB(Kaufmann et al. 2017). Similarly, in a case series by Plazzi et al. 4 out of 10 patients initially diagnosed with PAF were eventually diagnosed with MSA 5–7 years later(Plazzi et al. 1998). Those who were later diagnosed with MSA suffered with concomitant RBD, yet those who did not develop MSA were free from RBD. Additionally, 3 of the 4 MSA patients developed RBD symptoms shortly after or together with symptoms of autonomic failure, consistent with other reports describing a shorter interval of 1–5 years(Iranzo et al. 2013).
In a series of 8 PAF patients published by Miglis et al., 63 % met criteria for RBD, and all reported an autonomic symptom duration of at least 5 years without motor or cerebellar findings, making the progression to MSA unlikely(Miglis et al. 2017). Additionally, all 8 of these patients developed symptoms of dream enactment after developing autonomic failure, with an average time delay of 7.1 years. The authors noted that this may indicate a less aggressive brainstem involvement in PAF as compared to MSA(Miglis et al. 2017). Importantly, the timing of RBD symptoms relative to the emergence of autonomic symptoms may be useful to help distinguish these conditions, and, in fact, may provide a clue as to the likelihood of progression to an alpha-synucleinopathy(Miglis et al. 2017).
Factors in Prediction of Phenoconversion to Alpha-Synucleinopathy
Across multiple studies in independent cohorts there is consistent, although variable, association of RBD with risk of developing alpha-synucleinopathy(Hogl and Stefani 2017). Several longitudinal studies of patients with RBD have now demonstrated conversion to neurodegenerative syndromes. Schenck and colleagues(Schenck et al. 1996, Iranzo et al. 2013) reported that PD had developed in 11 (38%) of 29 patients with RBD within 4 years of the diagnosis(Iranzo et al. 2016). The majority (81%) of patients from the original cohort had been diagnosed with PD, DLB, or MSA 16 years later(Schenck et al. 2013). A different study demonstrated that the majority of 44 patients with RBD were diagnosed with PD, DLB, MSA, or mild cognitive impairment, with the risk of 35% at 5 years, of 73% at 10 years, and 92% at 14 years; and yet those who remained disease-free demonstrated findings such as decreased striatal dopamine transporter uptake or hyposmia, suggestive of -synucleinopathy(Iranzo et al. 2013, Iranzo et al. 2016). Similar results have been found in other cohorts(Wing et al. 2012, Postuma et al. 2015, Postuma et al. 2015, Youn et al. 2016), and the risk for phenoconversion from RBD to alpha-synucleinopathy increases with time following RBD diagnosis, with a median age of alpha-synucleinopathy diagnosis of approximately 75 years(Iranzo et al. 2016). Finally, a multi-center post-mortem study demonstrated that the overwhelming majority (98%) of 80 RBD patients suffering with parkinsonism or dementia had alpha-synuclein pathology in their brains(Boeve et al. 2013, Iranzo et al. 2016).
The diagnosis of alpha-synucleinopathies is based on the presence of specific motor and cognitive symptoms, but some patients develop a variety of other symptoms over a period of years during which the neurodegenerative process has been theorized to have already begun(Tolosa and Pont-Sunyer 2011, Donaghy et al. 2015, Iranzo et al. 2016). For example, RBD patients with a 2.5–5 year risk of phenoconversion to an alpha-synucleinopathy have been found to display substantia nigra hyperechogenicity, a reduction in dopamine transporter uptake in the striatum, hyposmia, and dysfunction of color vision perception(Iranzo et al. 2010, Postuma et al. 2011, Iranzo et al. 2016). In these cases, neurodegeneration can be monitored through dopamine transporter imaging (a progressive decline in striatal dopamine binding has been demonstrated), and through serial neuropsychological testing (which may demonstrate progressive deficits)(Iranzo et al. 2011, Terzaghi et al. 2013, Iranzo et al. 2016).
The manifestation of parkinsonism symptoms in patients with RBD who develop alpha-synucleinopathy is usually found to begin with hypomimia, hypophonia, and reduced arm swing, and of the akinetic-rigid subtype, as opposed to the tremor dominant subtype(Postuma et al. 2012). Most RBD patients who phenoconvert to PD do not present with resting tremor, but rather report complaints of motor slowness and shuffling gait with short steps(Iranzo et al. 2016). Facial akinesia, hypophonia, and reduced arm swing, followed by rigidity and akinesia are the first parkinsonian signs to appear, based on serial neurological examination(Postuma et al. 2012), whereas dementia, visual hallucinations, and delusions might appear several years following the diagnosis of PD(Iranzo et al. 2013).
It is difficult to predict which alpha-synucleiopathy disease that a patient with RBD will develop(Iranzo et al. 2016); however, clues exist, such as the fact that PD and DLB occur more frequently than MSA, and that the coexistence of nocturnal stridor is usually indicative of phenoconversion to MSA(Iranzo et al. 2016). Predictors of conversion to neurodegenerative diseases have been identified in longitudinal studies of those with RBD(Iranzo et al. 2010, Iranzo et al. 2011, Postuma et al. 2011, Postuma et al. 2012, Holtbernd et al. 2014, Postuma et al. 2015), and were similar between dementia-first and parkinsonism-first convertors(Postuma et al. 2011, Postuma et al. 2012). However, the onset of dementia in DLB is often preceded by mild cognitive impairment, a period lasting several years, during which time this is the major complaint(Iranzo et al. 2016). Although DLB and PD are overlapping processes(Berg et al. 2014), it has been suggested there are differences in mechanisms in those who develop dementia as their first neurodegenerative syndrome versus those who develop a primary parkinsonism, and an identification of specific markers for these different clinical syndromes in RBD would be quite important(Marchand et al. 2016).
Cross-sectional cognitive studies in RBD patients have demonstrated impaired attention and executive functions, episodic memory, and visuospatial abilities(Gagnon et al. 2012), and that approximately 50% of RBD patients have mild cognitive impairment (MCI), which is considered a risk factor for the development of dementia in PD and for DLB(Gagnon et al. 2012). In a prospective study of 76 RBD patients, followed over 3.6 years, Marchand et al found a distinct cognitive profile in patients who developed DLB: RBD patients who developed dementia first had poorer performance at baseline on all cognitive domains, and had a higher proportion of MCI at baseline, whereas RBD patients who developed parkinsonism first were similar to those who remained disease-free on cognitive tests performance and MCI diagnosis frequency(Marchand et al. 2016). They concluded that proper cognitive testing can be used to predict conversion subtypes, which could be used in future clinical trials in RBD in order to determine the impact of different interventions on cognitive decline(Marchand et al. 2016).
Three longitudinal studies on cognition have been performed in those with RBD(Fantini,et al. 2011, Terzaghi et al. 2013, Youn et al. 2016), which found deterioration in cognitive function, consistent with progression of neurodegeneration. It is not clear, however, if there is a baseline profile in RBD patients associated with primary conversion into dementia, as well as the optimal neurocognitive tests for early detection of prodromal DLB in RBD(Marchand et al. 2016). These three longitudinal studies indicate that there is a deterioration in cognition in RBD patients over time, especially in visual attention and visuospatial abilities. Relatively small sample sizes, however, did not allow statistical comparisons between patients who converted and those who remained disease-free(Fantini et al. 2011, Terzaghi et al. 2013), or between conversion subtypes (dementia-first vs. parkinsonism-first patients)(Youn et al. 2016).
The first of these studies followed 24 RBD patients and 12 healthy subjects for 2 years, and found worse delayed verbal memory and visuospatial abilities in patients at baseline and at follow-up, whereas worse visuospatial attention was also observed in patients at follow-up only(Fantini et al. 2011). The second study followed 20 RBD patients for 3.6 years, and found worse cognitive performance in 45% of patients (mostly in visuospatial abilities) and worse scores on non-verbal logic and attentional measures(Terzaghi et al. 2013). The most recent study followed 84 RBD patients for an average of 50.8 months, and 18 had converted at follow-up, which included 1 patient with spinocerebellar ataxia, 10 with parkinsonism first (9 PD, 1 MSA), and 7 with dementia first (4 DLB, 3 Alzheimer’s disease). Only visual attention at baseline differentiated between patients who developed a neurodegenerative disease from disease-free patients(Youn et al. 2016).
The exact pathophysiology of cognitive impairment in those RBD is unclear at this point, but it has been suggested to be due to a combination of subcortical and cortical dysfunctions(Marchand et al. 2016); structural neuroimaging studies have found cortical thinning, gray matter changes, and white matter anomalies in frontal areas, posterior regions, the substantia nigra, and brainstem structures(Unger et al. 2010, Scherfler et al. 2011, Rahayel et al. 2015, De Marzi et al. 2016, Ehrminger et al. 2016). MCI is a strong risk factor for dementia in RBD(Marchand et al. 2016), and heterogeneous MCI subtypes in RBD have been described, with attention/executive functions and visuospatial abilities as the main cognitive domains impaired(Gagnon et al. 2009, Molano et al. 2010, Terzaghi et al. 2013). Hypoperfusion on resting single-photon emission computerized tomography, mainly in posterior regions, along with more severe and widespread EEG slowing was demonstrated in RBD patients with concomitant MCI(Vendette et al. 2012, Rodrigues Brazete et al. 2013).
Marchand et al demonstrated that cognitive deficits reported in RBD are seen mostly in patients with dementia risk, and cognitive tests assessing attention and executive functions are useful in early detection of DLB in RBD patients(Marchand et al. 2016). The pattern of verbal-learning deficits in RBD patients reported by Marchand(Marchand et al. 2016) is similar to that reported in PD and mild DLB patients(Costa et al. 2014, Petrova et al. 2016). Adding a visuospatial task to the neuropsychological assessment increases the ability to predict the conversion to dementia in RBD patients, which is consistent with the prominent attention/executive functions and visuospatial declines noted early in DLB patients and in PD patients at risk of dementia(Goldman and Postuma 2014). Other studies demonstrate that patients who convert to DLB have poorer performance on cognitive tests measuring visuospatial functions at baseline, and hypometabolism in the parietal and occipital regions (visuospatial abilities)(Fujishiro et al. 2013, Cagnin et al. 2015). Finally, it has been shown that olfactory dysfunction could predict conversion into Lewy body disease(Mahlknecht et al. 2015),(Mahlknecht et al. 2016).
Despite these clinical and research factors, there are post-mortem reports of RBD patients without phenoconversion, despite the presence of autonomic dysfunction and alpha-synuclein pathology. For example, there is a case report of an RBD patient who developed constipation, hyposmia, depression, and mild cognitive impairment, but not parkinsonism or dementia, and yet post-mortem examination demonstrated neuronal loss and alpha-synuclein deposits in the cardiac and myenteric plexus along with Lewy bodies in the olfactory bulb, medulla, pons, substantia nigra, nucleus basalis of Meynert, and amygdala (but not in the neocortex)(Iranzo et al. 2014, Iranzo et al. 2016).
Similarly, in another case report of a patient with RBD, the presence of nocturnal stridor, and dysautonomic features, but without motor abnormalities, the post-mortem examination revealed the presence of alpha-synuclein-positive glial cytoplasmic inclusions in the brain, which would be typical of MSA(Gaig et al. 2008, Iranzo et al. 2016, Vilas et al. 2016). Clinicians and researchers should not consider all RBD patients with MCI as having a neurodegenerative disease, as further studies are needed to better characterize the evolution of different MCI subtypes in the RBD population and to determine their phenotype and their predictive value for dementia(Marchand et al. 2016).
In a four-year follow-up investigation, the International RBD Study Group evaluated 279 RBD patients from 12 centers who had initially participated in a questionnaire study. Patients who converted were older. Neither caffeine, nor smoking, nor alcohol exposure was able to predict conversion. Unexpectedly, converted patients were less likely to have been exposed to pesticides and were more likely to have a family history of dementia. Motor and autonomic symptoms were also more frequently observed in converted patients, and among converted patients with dementia, clonazepam use was more frequent(Postuma et al. 2015). Also discovered by the study group was an unexpected association with lifetime use of antidepressants in patients with IRBD, an effect that was stronger than the association with depression alone. Furthermore, an association with ischemic heart disease was also observed(Frauscher et al. 2014).
Clinical Approach to RBD
The first questionnaire tool developed for RBD was the 13-item Stiasny-Kolster RBD-Screening Questionnaire (RBDSQ), which demonstrated 96% sensitivity, but only 56% specificity(Miyamoto et al. 2009). A Japanese version of this questionnaire demonstrated a sensitivity of 88.5% and specificity of 90.9% in apnea patients (and 96.9% in healthy subjects), and in PD patients a sensitivity of 84% and specificity of 96%(Nomura et al. 2011). The RBD-HK (Hong Kong) scale includes 13 questions, with two frequency assessments for each question (lifetime and 1-year frequency), with a sensitivity of 82% and 87% specificity(Li et al. 2010). Most recently, the RBD Single-Question Screen (RBD1Q) was developed, which consists of a simple yes/no response to the question “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?” It was found to have a sensitivity of 93.8% and a specificity of 87.2%, and is thus considered to reliably detect disease(Postuma et al. 2012).
RBD in some cases places patients and their sleep partners at risk of physical injury during acting out of dreams(Ramos-Campoy et al. 2017). Self-inflicted injuries are reported to occur in 38–59% of patients (especially prior to treatment), and are usually resulting from hitting a wall or nightstand, or jumping out of bed(McCarter et al. 2014, Fernandez-Arcos et al. 2016). Whereas mild bruises, lacerations, and sprains are the most frequent injuries seen, extreme cases include dislocations, fractures, and rarely subdural hematomas(McCarter et al. 2014, Fernandez-Arcos et al. 2016).
The goal for symptomatic treatment of RBD is to reduce injury, but a sound basis of evidence for this remains lacking, and randomized controlled treatment trials are needed. Melatonin dosed 3–12 mg at bedtime is considered as the first-line therapy as there are few side effects(McCarter et al. 2013). Clonazepam 0.25–2.0 mg at bedtime can be used if initial melatonin is ineffective or intolerable, to decrease the occurrence of sleep-related injury caused by RBD. It should be used in caution in patients with dementia, gait disorders, or obstructive sleep apnea, and requires monitoring carefully over time(Aurora et al. 2010).
Pramipexole may be considered to treat RBD as a second-line therapy, but efficacy studies have shown contradictory results. Similarly, paroxetine or L-DOPA have not been conclusively demonstrated to improve RBD, and some studies suggesting that they may actually induce or exacerbate RBD. Limited data exists regarding the efficacy of acetylcholinesterase inhibitors, such as donepezil; however, they may be considered in those with a concomitant synucleinopathy(Aurora et al. 2010).
Other potential second- and third-line therapies include zolpidem, zopiclone, ramelteon, agomelatine, cannabinoids, benzodiazepines other than clonazepam, Yi-Gan San (traditional Chinese herbal medicine), desipramine, clozapine, carbamazepine, and sodium oxybate, all of which have anecdotal evidence only(Aurora et al. 2010, Jung and St Louis 2016). Other novel non-pharmacological approaches include a bed alarm system(Howell et al. 2011) and hypnosis(Larsen et al. 2016) (especially in those with psychiatric RBD), although these require further study(Jung and St Louis 2016).
Whereas the reduction of potentially injurious sleep behaviors in those with RBD is the priority in the majority of cases, others seek advice about the nature and long-term consequences of the disorder. Unfortunately, counseling and management guidelines do not yet exist for the care of those with RBD; in particular, parameters regarding what information the patient should receive regarding potential phenoconversion to an alpha-synucleinopathy have not been established(Iranzo et al. 2016). However, one approach put forth is to inform patients that, if certain symptoms arise, such as motor slowness or memory problems, they should consult their general practitioner, neurologist, or sleep specialist(Iranzo et al. 2016).
Ancillary testing, including neuroimaging, smell tests, or neuropsychological assessment, have, at this time, been deemed unnecessary because the results of such testing would not modify patient management(Iranzo et al. 2016). However, these instruments do have utility for research and will, ideally, be of clinical value in aiding risk stratification in the future(Fantini et al. 2006).
Discussion with Patients
The decision to discuss neurodegenerative risk and/or a prodromal disease diagnosis to a patient raises several ethical issues; unfortunately, systematic study of prognostic counseling in IRBD has been limited(Iranzo et al. 2016). Health care professionals must give patients sufficient information to enable them to make decisions that are adequately informed, and the disclosure of a diagnosis of a prodromal disease could have significant influence in patient decision-making for the future, as well as potentially seek out treatment and interventions that could alter the course of the disease(Arnaldi et al. 2017).
Unfortunately, there is a lack of neuroprotective therapy for synucleinopathies, making the benefit of prompt diagnosis and counseling relatively modest in IRBD patients, especially when viewed in the context of that being aware of the risk of developing a neurodegenerative disease could be potentially harmful from a psychological perspective(Arnaldi et al. 2017).
Whether a practitioner should disclose prognostic information regarding phenoconversion risk to an idiopathic RBD patient, or perhaps even more problematically, to the patient with isolated RSWA, remains unclear, and there may be relative advantages or disadvantages to full disclosure vs. watchful waiting without disclosure with any individual patient(Arnaldi et al. 2017). In their comprehensive review of this complicated subject, Arnaldi et al posit that general disclosure of prognostic risk is usually appropriate, but must be balanced appropriately by existing uncertainties(Arnaldi et al. 2017).
Legal Implications in RBD
Physicians are increasingly being called upon to consider sleep as a possible contributory factor in legal proceedings, but there exists little evidence to support opinion in court, and guidelines for assessing someone suspected of committing a crime in the context of a parasomnia or during sleep are non-existent(Morrison et al. 2014).
When faced with a violent or illegal act said to arise from or occur during sleep, physicians should first establish whether the behavior was due to RBD or another parasomnia, nocturnal dissociative episode, malingering or a functional disorder (including Munchhausen’s)(Morrison et al. 2014). The second step is to determine whether the behavior was the result of external or internal factors (pre-existing medical, psychological or psychiatric illness). It is then for the courts to answer the legal question of whether the disorder amounts to insanity or non-insane automatism(Wing et al. 2012). Violence during sleep in dementia, therefore, may result directly from the presence of RBD and this should be borne in mind when assessing the patient presenting with such a history(Morrison et al. 2014).
In RBD, the patient’s eyes are usually closed, (in contrast to disorders of arousal where the eyes are usually reported as being open), and they appear to interact with their dream rather than their immediate environment, and the behaviors are usually restricted to the bedroom(Morrison et al. 2014). The behaviors are often brief and, if awoken, the individual is orientated with a recollection of a dream that corresponds to the behavior(Schenck et al. 2009). Although RBD can be aggressive or violent, this does not appear to correlate with the waking personality(Fantini et al. 2005).
Conclusion and Future Directions
RBD is a sleep disorder that deserves attention for many reasons. Symptomatic treatments may be beneficial, as well as adaptive strategies for patients and sleep partners. However, although many with RBD will not experience future development of other, neurodegenerative conditions, the seriousness of this possibility and its implications has led to an increasing focus on this association.
A new diagnosis of RBD should therefore prompt a search for existing subtle signs of neurodegenerative disorders. Unfortunately, at this time, there is no established means of estimating the risk of future development of a neurodegenerative condition in a given individual, making it impossible to provide adequate guidance and counseling, and furthermore no effective therapies have yet been established that slow the neurodegenerative process in individuals with PD, DLB, or MSA. The reason for this lack has been postulated that the neurodegenerative process is too advanced for intervention by the time of diagnosis(Lang et al. 2013, Postuma et al. 2013). Therefore, RBD patients represent a potentially target-rich population for the testing of neuroprotective agents that would purportedly delay or stop the emergence of symptoms(Iranzo et al. 2016).
Further study of the RBD patient population would allow for an understanding of the range of severities of this condition between the sexes and across age groups, the rate of development of dementia in patients with mild cognitive impairment, and the identification of markers of neurodegeneration(Iranzo et al. 2016). For example, it has recently been reported that in patients with IRBD, glucocerebrosidase (GBA) gene variants are found more frequently than in controls, and are associated with impending PD and DLB; thus, IRBD patients carrying GBA variants could be studied with disease-modifying interventions aiming to restore the GBA metabolic pathway(Gamez-Valero et al. 2018). Additionally, patients with IRBD were recently found to have increased microglial activation in the substantia nigra along with reduced dopaminergic function in the putamen as detected through PET; but whether the presence of activated microglia in patients with IRBD represents a marker of short-term conversion to a clinically defined synucleinopathy in the near future remains to be determined(Stokholm et al. 2017). Other important clinical information that has yet to be discovered includes why RBD seems to predominate in men, the etiology of this disorder in those under 50 years of age(Lloyd et al. 2012), the role of antidepressant drugs as a precipitant of RBD.
Fortunately, there are now several observational cohort studies being undertaken, for example individuals with RBD are enrolling as part of the Parkinson’s Progression Markers Initiative (PPMI) study (http://www.ppmi-info.org); improved recognition of RBD may be useful in earlier diagnosis of the alpha-synuncleinopathies, in which early misdiagnosis is common(Adler et al. 2014). We suggest that those who are at higher risk of synucleinopathy also have associated risk factors, and/or signs and symptoms of a neurodegenerative disorder, that might be considered to be “RBD-plus”. Indeed, a recent study of 171 patients with RBD found widespread subtle impairment across multiple neuropsychiatric parameters, that are likely relevant to risk stratification(Barber et al. 2017). Based on a combination of testing and clinical findings, we suggest the term “RBD-plus” to such designate patients who may have a preclinical version of an alpha-synucleinopathy.
Finally, perhaps the most important need for research into this area of sleep and neurology is the discovery and testing of biomarkers to determine in whom and when a conversion to alpha-synuncleinopathy would occur, and of a neuroprotective strategy to prevent ongoing neurodegeneration in identified at-risk individuals(Iranzo et al. 2016).
Highlights.
Rapid eye movement behavior disorder (RBD) is a well-described parasomnia, summarized here.
RBD is associated with the development of the so-called alpha-synucleinopathies.
We review this association, as well as treatment options for the symptoms of RBD.
Acknowledgments
Declarations for each author:
• Daniel Barone
o Financial support (presence or absence): private grant support
o Off-label or investigational use: none
o Conflict of interest (presence or absence): none
• Claire Henchcliffe
o Financial support (presence or absence): grant support from NIH/NINDS
o Off-label or investigational use: none
o Conflict of interest (presence or absence): none
Abbreviations
- DLB
dementia with Lewy bodies
- EEG
electroencephalogram
- IRBD
idiopathic RBD
- LC
locus coeruleus
- LDT
laterodorsal tegmentum
- MCI
mild cognitive impairment
- MRF
mesencephalic reticular formation
- MSA
multiple system atrophy
- Msec
millisecond
- PD
Parkinson’s disease
- PGO
pontogeniculooccipital
- PPT
pedunculopontine tegmentum
- PRF
pontine reticular formation
- PSG
polysomnogram
- RBD
REM sleep behavior diorder
- REM
Rapid eye movement
- RSWA
REM sleep without atonia
- UPDRS
Unified Parkinson Disease Rating Scale
References
- Adler CH, Beach TG, Hentz JG, Shill HA, Caviness JN, Driver-Dunckley E, et al. (2014). “Low clinical diagnostic accuracy of early vs advanced Parkinson disease: clinicopathologic study.” Neurology 83(5): 406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguirre-Mardones C, Iranzo A, Vilas D, Serradell M, Gaig C, Santamaria J, et al. (2015). “Prevalence and timeline of nonmotor symptoms in idiopathic rapid eye movement sleep behavior disorder.” J Neurol 262(6): 1568–1578. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine (2014. ). “International Classification of Sleep Disorders, 3rd ed.” (Darien, IL: ). [Google Scholar]
- Applebee GA, Attarian HP and Schenck CH (2009). “An angry bed partner.” J Clin Sleep Med 5(5): 477–479. [PMC free article] [PubMed] [Google Scholar]
- Arnaldi D, Antelmi E, St Louis EK, Postuma RB and Arnulf I (2017). “Idiopathic REM sleep behavior disorder and neurodegenerative risk: To tell or not to tell to the patient? How to minimize the risk?” Sleep Med Rev 36: 82–95. [DOI] [PubMed] [Google Scholar]
- Arnulf I (2012). “REM sleep behavior disorder: motor manifestations and pathophysiology.” Mov Disord 27(6): 677–689. [DOI] [PubMed] [Google Scholar]
- Aserinsky E and Kleitman N (1953). “Regularly occurring periods of eye motility, and concomitant phenomena, during sleep.” Science 118(3062): 273–274. [DOI] [PubMed] [Google Scholar]
- Aurora RN, Zak RS, Maganti RK, Auerbach SH, Casey KR, Chowdhuri S, et al. ; Standards of Practice Committee; American Academy of Sleep Medicine. (2010). “Best practice guide for the treatment of REM sleep behavior disorder (RBD).” J Clin Sleep Med 6(1):85–95. [PMC free article] [PubMed] [Google Scholar]
- Baghdoyan HA, Rodrigo-Angulo ML, McCarley RW and Hobson JA (1984). “Site-specific enhancement and suppression of desynchronized sleep signs following cholinergic stimulation of three brainstem regions.” Brain Res 306(1–2): 39–52. [DOI] [PubMed] [Google Scholar]
- Barber TR, Lawton M, Rolinski M, Evetts S, Baig F, Ruffmann C, et al. (2017). “Prodromal Parkinsonism and Neurodegenerative Risk Stratification in REM Sleep Behavior Disorder.” Sleep 40(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barone DA, Ebben MR, Samie A, Mortara D and Krieger AC (2015). “Autonomic dysfunction in isolated rapid eye movement sleep without atonia.” Clin Neurophysiol 126(4): 731–735. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Dugger BN, Sandroni P, Parisi JE and Low PA (2009). “Dopamine cell loss in the periaqueductal gray in multiple system atrophy and Lewy body dementia.” Neurology 73(2): 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg D, Postuma RB, Bloem B, Chan P, Dubois B, Gasser T, et al. (2014). “Time to redefine PD? Introductory statement of the MDS Task Force on the definition of Parkinson’s disease.” Mov Disord 29(4): 454–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornara KA, Dietrichs E and Toft M (2013). “REM sleep behavior disorder in Parkinson’s disease--is there a gender difference?” Parkinsonism Relat Disord 19(1): 120–122. [DOI] [PubMed] [Google Scholar]
- Blumberg MS and Lucas DE (1994). “Dual mechanisms of twitching during sleep in neonatal rats.” Behav Neurosci 108(6): 1196–1202. [DOI] [PubMed] [Google Scholar]
- Boeve BF (2010). “REM sleep behavior disorder: Updated review of the core features, the REM sleep behavior disorder-neurodegenerative disease association, evolving concepts, controversies, and future directions.” Ann N Y Acad Sci 1184: 15–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Kokmen E, Smith GE, Ivnik RJ, et al. (1998). “REM sleep behavior disorder and degenerative dementia: an association likely reflecting Lewy body disease.” Neurology 51(2): 363–370. [DOI] [PubMed] [Google Scholar]
- Boeve BF, Dickson DW, Olson EJ, Shepard JW, Silber MH, Ferman TJ, et al. (2007). “Insights into REM sleep behavior disorder pathophysiology in brainstem-predominant Lewy body disease.” Sleep Med 8(1): 60–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Ferman TJ, Lin SC, Benarroch EE, Schmeichel AM, et al. (2013). “Clinicopathologic correlations in 172 cases of rapid eye movement sleep behavior disorder with or without a coexisting neurologic disorder.” Sleep Med 14(8): 754–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boeve BF, Silber MH, Saper CB, Ferman TJ, Dickson DW, Parisi JE, et al. (2007). “Pathophysiology of REM sleep behaviour disorder and relevance to neurodegenerative disease.” Brain 130(Pt 11): 2770–2788. [DOI] [PubMed] [Google Scholar]
- Boissard R, Fort P, Gervasoni D, Barbagli B and Luppi PH (2003). “Localization of the GABAergic and non-GABAergic neurons projecting to the sublaterodorsal nucleus and potentially gating paradoxical sleep onset.” Eur J Neurosci 18(6): 1627–1639. [DOI] [PubMed] [Google Scholar]
- Boissard R, Gervasoni D, Schmidt MH, Barbagli B, Fort P and Luppi PH (2002). “The rat ponto-medullary network responsible for paradoxical sleep onset and maintenance: a combined microinjection and functional neuroanatomical study.” Eur J Neurosci 16(10): 1959–1973. [DOI] [PubMed] [Google Scholar]
- Bonakis A, Howard RS, Ebrahim IO, Merritt S and Williams A (2009). “REM sleep behaviour disorder (RBD) and its associations in young patients.” Sleep Med 10(6): 641–645. [DOI] [PubMed] [Google Scholar]
- Boot BP, Boeve BF, Roberts RO, Ferman TJ, Geda YE, Pankratz VS, et al. (2012). “Probable rapid eye movement sleep behavior disorder increases risk for mild cognitive impairment and Parkinson disease: a population-based study.” Ann Neurol 71(1): 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN and Braak E (2003). “Staging of brain pathology related to sporadic Parkinson’s disease.” Neurobiol Aging 24(2): 197–211. [DOI] [PubMed] [Google Scholar]
- Brooks PL and Peever JH (2008). “Glycinergic and GABA(A)-mediated inhibition of somatic motoneurons does not mediate rapid eye movement sleep motor atonia.” J Neurosci 28(14): 3535–3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks PL and Peever JH (2012). “Identification of the transmitter and receptor mechanisms responsible for REM sleep paralysis.” J Neurosci 32(29): 9785–9795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess C, Lai D, Siegel J and Peever J (2008). “An endogenous glutamatergic drive onto somatic motoneurons contributes to the stereotypical pattern of muscle tone across the sleep-wake cycle.” J Neurosci 28(18): 4649–4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagnin A, Busse C, Gardini S, Jelcic N, Guzzo C, Gnoato F, et al. (2015). “Clinical and Cognitive Phenotype of Mild Cognitive Impairment Evolving to Dementia with Lewy Bodies.” Dement Geriatr Cogn Dis Extra 5(3): 442–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chase MH (2013). “Motor control during sleep and wakefulness: clarifying controversies and resolving paradoxes.” Sleep Med Rev 17(4): 299–312. [DOI] [PubMed] [Google Scholar]
- Chase MH and Morales FR (1982). “Phasic changes in motoneuron membrane potential during REM periods of active sleep.” Neurosci Lett 34(2): 177–182. [DOI] [PubMed] [Google Scholar]
- Chase MH and Morales FR (1983). “Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep.” Science 221(4616): 1195–1198. [DOI] [PubMed] [Google Scholar]
- Chen MC, Yu H, Huang ZL and Lu J (2013). “Rapid eye movement sleep behavior disorder.” Curr Opin Neurobiol 23(5): 793–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiaro G, Calandra-Buonaura G, Cecere A, Mignani F, Sambati L, Loddo G, et al. (2017). “REM sleep behavior disorder, autonomic dysfunction and synuclein-related neurodegeneration: where do we stand?” Clin Auton Res doi: 10.1007/s10286-017-0460-4. [DOI] [PubMed] [Google Scholar]
- Classen J and Schnitzler A (2010). “What does the pedunculopontine nucleus do?” Neurology 75(11): 944–945. [DOI] [PubMed] [Google Scholar]
- Clement O, Sapin E, Berod A, Fort P and Luppi PH (2011). “Evidence that neurons of the sublaterodorsal tegmental nucleus triggering paradoxical (REM) sleep are glutamatergic.” Sleep 34(4): 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cochen V, Arnulf I, Demeret S, Neulat ML, Gourlet V, Drouot X, et al. (2005). “Vivid dreams, hallucinations, psychosis and REM sleep in Guillain-Barre syndrome.” Brain 128(Pt 11): 2535–2545. [DOI] [PubMed] [Google Scholar]
- Compta Y, Iranzo A, Santamaria J, Casamitjana R and Graus F (2007). “REM sleep behavior disorder and narcoleptic features in anti-Ma2-associated encephalitis.” Sleep 30(6): 767–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa A, Monaco M, Zabberoni S, Peppe A, Perri R, Fadda L, et al. (2014). “Free and cued recall memory in Parkinson’s disease associated with amnestic mild cognitive impairment.” PLoS One 9(1): e86233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culebras A and Moore JT (1989). “Magnetic resonance findings in REM sleep behavior disorder.” Neurology 39(11): 1519–1523. [DOI] [PubMed] [Google Scholar]
- Dauvilliers Y, Postuma RB, Ferini-Strambi L, Arnulf I, Hogl B, Manni R, et al. (2013). “Family history of idiopathic REM behavior disorder: a multicenter case-control study.” Neurology 80(24): 2233–2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dauvilliers Y, Rompre S, Gagnon JF, Vendette M, Petit D and Montplaisir J (2007). “REM sleep characteristics in narcolepsy and REM sleep behavior disorder.” Sleep 30(7): 844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Marzi R, Seppi K, Hogl B, Muller C, Scherfler C, Stefani A, et al. (2016). “Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder.” Ann Neurol 79(6): 1026–1030. [DOI] [PubMed] [Google Scholar]
- De Cock VC, Lannuzel A, Verhaeghe S, Roze E, Ruberg M, Derenne JP, et al. (2007). “REM sleep behavior disorder in patients with guadeloupean parkinsonism, a tauopathy.” Sleep 30(8): 1026–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dement W and Kleitman N (1957). “Cyclic variations in EEG during sleep and their relation to eye movements, body motility, and dreaming.” Electroencephalogr Clin Neurophysiol 9(4): 673–690. [DOI] [PubMed] [Google Scholar]
- Dement W and Kleitman N (1957). “The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming.” J Exp Psychol 53(5): 339–346. [DOI] [PubMed] [Google Scholar]
- Donaghy PC, O’Brien JT and Thomas AJ (2015). “Prodromal dementia with Lewy bodies.” Psychol Med 45(2): 259–268. [DOI] [PubMed] [Google Scholar]
- Ebben MR, Shahbazi M, Lange DJ and Krieger AC (2012). “REM behavior disorder associated with familial amyotrophic lateral sclerosis.” Amyotroph Lateral Scler 13(5): 473–474. [DOI] [PubMed] [Google Scholar]
- Ehrminger M, Latimier A, Pyatigorskaya N, Garcia-Lorenzo D, Leu-Semenescu S, Vidailhet M, et al. (2016). “The coeruleus/subcoeruleus complex in idiopathic rapid eye movement sleep behaviour disorder.” Brain 139(Pt 4): 1180–1188. [DOI] [PubMed] [Google Scholar]
- Fantini ML, Corona A, Clerici S and Ferini-Strambi L (2005). “Aggressive dream content without daytime aggressiveness in REM sleep behavior disorder.” Neurology 65(7): 1010–1015. [DOI] [PubMed] [Google Scholar]
- Fantini ML, Farini E, Ortelli P, Zucconi M, Manconi M, Cappa S, et al. (2011). “Longitudinal study of cognitive function in idiopathic REM sleep behavior disorder.” Sleep 34(5): 619–625. [PMC free article] [PubMed] [Google Scholar]
- Fantini ML, Postuma RB, Montplaisir J and Ferini-Strambi L (2006). “Olfactory deficit in idiopathic rapid eye movements sleep behavior disorder.” Brain Res Bull 70(4–6): 386–390. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Di Gioia MR, Castronovo V, Oldani A, Zucconi M and Cappa SF (2004). “Neuropsychological assessment in idiopathic REM sleep behavior disorder (RBD): does the idiopathic form of RBD really exist?” Neurology 62(1): 41–45. [DOI] [PubMed] [Google Scholar]
- Ferini-Strambi L, Oldani A, Zucconi M and Smirne S (1996). “Cardiac autonomic activity during wakefulness and sleep in REM sleep behavior disorder.” Sleep 19(5): 367–369. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Boeve B, Smith GE, Lin SC, Silber MH, Pedraza O, et al. (2011). “Inclusion of RBD improves the diagnostic classification of dementia with Lewy bodies.” Neurology 77(9): 875–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Arcos A, Iranzo A, Serradell M, Gaig C and Santamaria J (2016). “The Clinical Phenotype of Idiopathic Rapid Eye Movement Sleep Behavior Disorder at Presentation: A Study in 203 Consecutive Patients.” Sleep 39(1): 121–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri R, Franceschini C, Zucconi M, Vandi S, Poli F, Bruni O, et al. (2008). “Searching for a marker of REM sleep behavior disorder: submentalis muscle EMG amplitude analysis during sleep in patients with narcolepsy/cataplexy.” Sleep 31(10): 1409–1417. [PMC free article] [PubMed] [Google Scholar]
- Frauscher B, Jennum P, Ju YE, Postuma RB, Arnulf I, Cochen De Cock V, et al. (2014). “Comorbidity and medication in REM sleep behavior disorder: a multicenter case-control study.” Neurology 82(12): 1076–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujishiro H, Iseki E, Kasanuki K, Chiba Y, Ota K, Murayama N, et al. (2013). “A follow up study of non-demented patients with primary visual cortical hypometabolism: prodromal dementia with Lewy bodies.” J Neurol Sci 334(1–2): 48–54. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Bedard MA, Fantini ML, Petit D, Panisset M, Rompre S, et al. (2002). “REM sleep behavior disorder and REM sleep without atonia in Parkinson’s disease.” Neurology 59(4): 585–589. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Bertrand JA and Genier Marchand D (2012). “Cognition in rapid eye movement sleep behavior disorder.” Front Neurol 3: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon JF, Postuma RB, Mazza S, Doyon J and Montplaisir J (2006). “Rapid-eye-movement sleep behaviour disorder and neurodegenerative diseases.” Lancet Neurol 5(5): 424–432. [DOI] [PubMed] [Google Scholar]
- Gagnon JF, Vendette M, Postuma RB, Desjardins C, Massicotte-Marquez J, Panisset M, et al. (2009). “Mild cognitive impairment in rapid eye movement sleep behavior disorder and Parkinson’s disease.” Ann Neurol 66(1): 39–47. [DOI] [PubMed] [Google Scholar]
- Gaig C, Iranzo A, Tolosa E, Vilaseca I, Rey MJ and Santamaria J (2008). “Pathological description of a non-motor variant of multiple system atrophy.” J Neurol Neurosurg Psychiatry 79(12): 1399–1400. [DOI] [PubMed] [Google Scholar]
- Gamez-Valero A, Iranzo A, Serradell M, Vilas D, Santamaria J, Gaig C, et al. (2018). “Glucocerebrosidase gene variants are accumulated in idiopathic REM sleep behavior disorder.” Parkinsonism Relat Disord doi: 10.1016/j.parkreldis.2018.02.034. [DOI] [PubMed] [Google Scholar]
- Goldman JG and Postuma R (2014). “Premotor and nonmotor features of Parkinson’s disease.” Curr Opin Neurol 27(4): 434–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Choco MJ, Iranzo A, Blanco Y, Graus F, Santamaria J and Saiz A (2007). “Prevalence of restless legs syndrome and REM sleep behavior disorder in multiple sclerosis.” Mult Scler 13(6): 805–808. [DOI] [PubMed] [Google Scholar]
- Grillner S and Zangger P (1979). “On the central generation of locomotion in the low spinal cat.” Exp Brain Res 34(2): 241–261. [DOI] [PubMed] [Google Scholar]
- Hobson JA and Pace-Schott EF (2002). “The cognitive neuroscience of sleep: neuronal systems, consciousness and learning.” Nat Rev Neurosci 3(9): 679–693. [DOI] [PubMed] [Google Scholar]
- Hogl B and Stefani A (2017). “REM sleep behavior disorder (RBD): Update on diagnosis and treatment.” Somnologie (Berl) 21(Suppl 1): 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes CJ, Mainville LS and Jones BE (1994). “Distribution of cholinergic, GABAergic and serotonergic neurons in the medial medullary reticular formation and their projections studied by cytotoxic lesions in the cat.” Neuroscience 62(4): 1155–1178. [DOI] [PubMed] [Google Scholar]
- Holtbernd F, Gagnon JF, Postuma RB, Ma Y, Tang CC, Feigin A, et al. (2014). “Abnormal metabolic network activity in REM sleep behavior disorder.” Neurology 82(7): 620–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoque R and Chesson AL Jr. (2010). “Pharmacologically induced/exacerbated restless legs syndrome, periodic limb movements of sleep, and REM behavior disorder/REM sleep without atonia: literature review, qualitative scoring, and comparative analysis.” J Clin Sleep Med 6(1): 79–83. [PMC free article] [PubMed] [Google Scholar]
- Hossaini M, Goos JA, Kohli SK and Holstege JC (2012). “Distribution of glycine/GABA neurons in the ventromedial medulla with descending spinal projections and evidence for an ascending glycine/GABA projection.” PLoS One 7(4): e35293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell MJ, Arneson PA and Schenck CH (2011). “A novel therapy for REM sleep behavior disorder (RBD).” J Clin Sleep Med 7(6): 639–644a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain AM, Miller PP and Carwile ST (2001). “Rem sleep behavior disorder: potential relationship to post-traumatic stress disorder.” J Clin Neurophysiol 18(2): 148–157. [DOI] [PubMed] [Google Scholar]
- Iranzo A and Aparicio J (2009). “A lesson from anatomy: focal brain lesions causing REM sleep behavior disorder.” Sleep Med 10(1): 9–12. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Fernandez-Arcos A, Tolosa E, Serradell M, Molinuevo JL, Valldeoriola F, et al. (2014). “Neurodegenerative disorder risk in idiopathic REM sleep behavior disorder: study in 174 patients.” PLoS One 9(2): e89741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iranzo A, Gelpi E, Tolosa E, Molinuevo JL, Serradell M, Gaig C, et al. (2014). “Neuropathology of prodromal Lewy body disease.” Mov Disord 29(3): 410–415. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Lomena F, Stockner H, Valldeoriola F, Vilaseca I, Salamero M, et al. (2010). “Decreased striatal dopamine transporter uptake and substantia nigra hyperechogenicity as risk markers of synucleinopathy in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study [corrected].” Lancet Neurol 9(11): 1070–1077. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Molinuevo JL, Santamaria J, Serradell M, Marti MJ, Valldeoriola F, et al. (2006). “Rapid-eye-movement sleep behaviour disorder as an early marker for a neurodegenerative disorder: a descriptive study.” Lancet Neurol 5(7): 572–577. [DOI] [PubMed] [Google Scholar]
- Iranzo A and Santamaria J (2005). “Severe obstructive sleep apnea/hypopnea mimicking REM sleep behavior disorder.” Sleep 28(2): 203–206. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Santamaria J, Rye DB, Valldeoriola F, Marti MJ, Munoz E, et al. (2005). “Characteristics of idiopathic REM sleep behavior disorder and that associated with MSA and PD.” Neurology 65(2): 247–252. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Santamaria J and Tolosa E (2016). “Idiopathic rapid eye movement sleep behaviour disorder: diagnosis, management, and the need for neuroprotective interventions.” Lancet Neurol 15(4): 405–419. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Serradell M, Vilaseca I, Valldeoriola F, Salamero M, Molina C, et al. (2013). “Longitudinal assessment of olfactory function in idiopathic REM sleep behavior disorder.” Parkinsonism Relat Disord 19(6): 600–604. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Stockner H, Serradell M, Seppi K, Valldeoriola F, Frauscher B, et al. (2014). “Five-year follow-up of substantia nigra echogenicity in idiopathic REM sleep behavior disorder.” Mov Disord 29(14): 1774–1780. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Tolosa E, Gelpi E, Molinuevo JL, Valldeoriola F, Serradell M, et al. (2013). “Neurodegenerative disease status and post-mortem pathology in idiopathic rapid-eye-movement sleep behaviour disorder: an observational cohort study.” Lancet Neurol 12(5): 443–453. [DOI] [PubMed] [Google Scholar]
- Iranzo A, Valldeoriola F, Lomena F, Molinuevo JL, Serradell M, Salamero M, et al. (2011). “Serial dopamine transporter imaging of nigrostriatal function in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a prospective study.” Lancet Neurol 10(9): 797–805. [DOI] [PubMed] [Google Scholar]
- Jennum PJ, Pedersen LO, Bahl JM, Modvig S, Fog K, Holm A, et al. (2017). “Cerebrospinal fluid biomarkers of neurodegeneration are decreased or normal in narcolepsy.” Sleep 1;40(1). [DOI] [PubMed] [Google Scholar]
- Jiang H, Huang J, Shen Y, Guo S, Wang L, Han C, et al. (2017). “RBD and Neurodegenerative Diseases.” Mol Neurobiol 54(4):2997–3006 [DOI] [PubMed] [Google Scholar]
- Jouvet M (1962). “Recherches sur les structures nerveuses et les mecanismes responsables des differentes phases du sommeil physiologique.” Arch Ital Biol 100: 125–206. [PubMed] [Google Scholar]
- Jung Y and St Louis EK (2016). “Treatment of REM Sleep Behavior Disorder.” Curr Treat Options Neurol 18(11): 50. [DOI] [PubMed] [Google Scholar]
- Kang SH, Yoon IY, Lee SD, Han JW, Kim TH and Kim KW (2013). “REM sleep behavior disorder in the Korean elderly population: prevalence and clinical characteristics.” Sleep 36(8): 1147–1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann H, Norcliffe-Kaufmann L, Palma JA, Biaggioni I, Low PA, Singer W, et al. (2017). “Natural history of pure autonomic failure: A United States prospective cohort.” Ann Neurol 81(2): 287–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalil A, Wright MA, Walker MC and Eriksson SH (2013). “Loss of rapid eye movement sleep atonia in patients with REM sleep behavioral disorder, narcolepsy, and isolated loss of REM atonia.” J Clin Sleep Med 9(10): 1039–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Tachibana N, Kohyama J, Otsuka Y, Fukazawa S and Waki R (2000). “A discrete pontine ischemic lesion could cause REM sleep behavior disorder.” Neurology 55(6): 894–895. [DOI] [PubMed] [Google Scholar]
- Knudsen S, Gammeltoft S and Jennum PJ (2010). “Rapid eye movement sleep behaviour disorder in patients with narcolepsy is associated with hypocretin-1 deficiency.” Brain 133(Pt 2): 568–579. [DOI] [PubMed] [Google Scholar]
- Lai YY and Siegel JM (1988). “Medullary regions mediating atonia.” J Neurosci 8(12): 4790–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam SP, Zhang J and Wing YK (2013). “REM Sleep Behavior Disorder: From Epidemiology to Heterogeneity.” Sleep 36(8): 1117–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranchi PA, Fradette L, Gagnon JF, Colombo R and Montplaisir J (2007). “Cardiac autonomic regulation during sleep in idiopathic REM sleep behavior disorder.” Sleep 30(8): 1019–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang AE, Melamed E, Poewe W and Rascol O (2013). “Trial designs used to study neuroprotective therapy in Parkinson’s disease.” Mov Disord 28(1): 86–95. [DOI] [PubMed] [Google Scholar]
- Larsen V, Richardson J, Boeve BF, Silber MH, St EK. Louis. (2016). “Clinical characteristics and treatment outcomes of patients treated with hypnosis for parasomnias. “ Sleep 39(Suppl (abstract))(A237). [Google Scholar]
- Lee K, Baron K, Soca R and Attarian H (2016). “The Prevalence and Characteristics of REM Sleep without Atonia (RSWA) in Patients Taking Antidepressants.” J Clin Sleep Med 12(3): 351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard CS and Llinas R (1994). “Serotonergic and cholinergic inhibition of mesopontine cholinergic neurons controlling REM sleep: an in vitro electrophysiological study.” Neuroscience 59(2): 309–330. [DOI] [PubMed] [Google Scholar]
- Li SX, Wing YK, Lam SP, Zhang J, Yu MW, Ho CK, et al. (2010). “Validation of a new REM sleep behavior disorder questionnaire (RBDQ-HK).” Sleep Med 11(1): 43–48. [DOI] [PubMed] [Google Scholar]
- Limousin N, Dehais C, Gout O, Heran F, Oudiette D and Arnulf I (2009). “A brainstem inflammatory lesion causing REM sleep behavior disorder and sleepwalking (parasomnia overlap disorder).” Sleep Med 10(9): 1059–1062. [DOI] [PubMed] [Google Scholar]
- Lin FC, Liu CK and Hsu CY (2009). “Rapid-eye-movement sleep behavior disorder secondary to acute aseptic limbic encephalitis.” J Neurol 256(7): 1174–1176. [DOI] [PubMed] [Google Scholar]
- Lloyd R, Tippmann-Peikert M, Slocumb N and Kotagal S (2012). “Characteristics of REM sleep behavior disorder in childhood.” J Clin Sleep Med 8(2): 127–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Coco D, Caruso G and Mattaliano A (2009). “REM sleep behavior disorder in patients with DJ-1 mutations and parkinsonism-dementia-ALS complex.” Mov Disord 24(10): 1555–1556. [DOI] [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M and Saper CB (2006). “A putative flip-flop switch for control of REM sleep.” Nature 441(7093): 589–594. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Greene RW, Semba K, Kamondi A, McCarley RW and Reiner PB (1992). “Serotonin hyperpolarizes cholinergic low-threshold burst neurons in the rat laterodorsal tegmental nucleus in vitro.” Proc Natl Acad Sci U S A 89(2): 743–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahlknecht P, Iranzo A, Hogl B, Frauscher B, Muller C, Santamaria J, et al. (2015). “Olfactory dysfunction predicts early transition to a Lewy body disease in idiopathic RBD.” Neurology 84(7): 654–658. [DOI] [PubMed] [Google Scholar]
- Mahlknecht P, Pechlaner R, Boesveldt S, Volc D, Pinter B, Reiter E, et al. (2016). “Optimizing odor identification testing as quick and accurate diagnostic tool for Parkinson’s disease.” Mov Disord 31(9): 1408–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney KJ, Mainville L and Jones BE (1999). “Differential c-Fos expression in cholinergic, monoaminergic, and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery.” J Neurosci 19(8): 3057–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manni R, Ratti PL and Terzaghi M (2011). “Secondary “incidental” REM sleep behavior disorder: do we ever think of it?” Sleep Med 12 Suppl 2: S50–53. [DOI] [PubMed] [Google Scholar]
- Manni R, Terzaghi M and Glorioso M (2009). “Motor-behavioral episodes in REM sleep behavior disorder and phasic events during REM sleep.” Sleep 32(2): 241–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand DG, Montplaisir J, Postuma RB, Rahayel S and Gagnon JF (2016). “Detecting the cognitive prodrome of dementia with Lewy bodies: A prospective study of REM sleep behavior disorder.” Sleep doi: 10.1093/sleep/zsw014. [DOI] [PubMed] [Google Scholar]
- Mattarozzi K, Bellucci C, Campi C, Cipolli C, Ferri R, Franceschini C, et al. (2008). “Clinical, behavioural and polysomnographic correlates of cataplexy in patients with narcolepsy/cataplexy.” Sleep Med 9(4): 425–433. [DOI] [PubMed] [Google Scholar]
- McCarley RW, Greene RW, Rainnie D, and Portas CM (1995). “Brainstem neuromodulation and REM sleep.” Seminars in the Neurosciences 7(5): 341–354. [Google Scholar]
- McCarter SJ, Boswell CL, St Louis EK, Dueffert LG, Slocumb N, Boeve BF, et al. (2013). “Treatment outcomes in REM sleep behavior disorder.” Sleep Med 14(3): 237–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter SJ, St Louis EK, Boswell CL, Dueffert LG, Slocumb N, Boeve BF, et al. (2014). “Factors associated with injury in REM sleep behavior disorder.” Sleep Med 15(11): 1332–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglis MG, Muppidi S, During E and Jaradeh S (2017). “A case series of REM sleep behavior disorder in pure autonomic failure.” Clin Auton Res 27(1): 41–44. [DOI] [PubMed] [Google Scholar]
- Mitani A, Ito K, Hallanger AE, Wainer BH, Kataoka K and McCarley RW (1988). “Cholinergic projections from the laterodorsal and pedunculopontine tegmental nuclei to the pontine gigantocellular tegmental field in the cat.” Brain Res 451(1–2): 397–402. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Miyamoto M, Inoue Y, Usui Y, Suzuki K and Hirata K (2006). “Reduced cardiac 123I-MIBG scintigraphy in idiopathic REM sleep behavior disorder.” Neurology 67(12): 2236–2238. [DOI] [PubMed] [Google Scholar]
- Miyamoto T, Miyamoto M, Iwanami M, Kobayashi M, Nakamura M, Inoue Y, et al. (2009). “The REM sleep behavior disorder screening questionnaire: validation study of a Japanese version.” Sleep Med 10(10): 1151–1154. [DOI] [PubMed] [Google Scholar]
- Molano J, Boeve B, Ferman T, Smith G, Parisi J, Dickson D, et al. (2010). “Mild cognitive impairment associated with limbic and neocortical Lewy body disease: a clinicopathological study.” Brain 133(Pt 2): 540–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales FR and Chase MH (1982). “Repetitive synaptic potentials responsible for inhibition of spinal cord motoneurons during active sleep.” Exp Neurol 78(2): 471–476. [DOI] [PubMed] [Google Scholar]
- Morrison I, Rumbold JM and Riha RL (2014). “Medicolegal aspects of complex behaviours arising from the sleep period: a review and guide for the practising sleep physician.” Sleep Med Rev 18(3): 249–260. [DOI] [PubMed] [Google Scholar]
- Munhoz RP and Teive HA (2014). “REM sleep behaviour disorder: how useful is it for the differential diagnosis of parkinsonism?” Clin Neurol Neurosurg 127: 71–74. [DOI] [PubMed] [Google Scholar]
- Ng M and Pavlova M (2013). “Why are seizures rare in rapid eye movement sleep? Review of the frequency of seizures in different sleep stages.” Epilepsy Res Treat 2013: 932790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nightingale S, Orgill JC, Ebrahim IO, de Lacy SF, Agrawal S and Williams AJ (2005). “The association between narcolepsy and REM behavior disorder (RBD).” Sleep Med 6(3): 253–258. [DOI] [PubMed] [Google Scholar]
- Noga BR, Kettler J and Jordan LM (1988). “Locomotion produced in mesencephalic cats by injections of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip.” J Neurosci 8(6): 2074–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura T, Inoue Y, Kagimura T, Uemura Y and Nakashima K (2011). “Utility of the REM sleep behavior disorder screening questionnaire (RBDSQ) in Parkinson’s disease patients.” Sleep Med 12(7): 711–713. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Caulet M and Priest RG (1997). “Violent behavior during sleep.” J Clin Psychiatry 58(8): 369–376. [PubMed] [Google Scholar]
- Olson EJ, Boeve BF and Silber MH (2000). “Rapid eye movement sleep behaviour disorder: demographic, clinical and laboratory findings in 93 cases.” Brain 123 ( Pt 2): 331–339. [DOI] [PubMed] [Google Scholar]
- Oudiette D, Leu-Semenescu S, Roze E, Vidailhet M, De Cock VC, Golmard JL, et al. (2012). “A motor signature of REM sleep behavior disorder.” Mov Disord 27(3): 428–431. [DOI] [PubMed] [Google Scholar]
- Palma JA, Fernandez-Cordon C, Coon EA, Low PA, Miglis MG, Jaradeh S, et al. (2015). “Prevalence of REM sleep behavior disorder in multiple system atrophy: a multicenter study and meta-analysis.” Clin Auton Res 25(1): 69–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parish JM (2007). “Violent dreaming and antidepressant drugs: or how paroxetine made me dream that I was fighting Saddam Hussein.” J Clin Sleep Med 3(5): 529–531. [PMC free article] [PubMed] [Google Scholar]
- Peever J, Luppi PH and Montplaisir J (2014). “Breakdown in REM sleep circuitry underlies REM sleep behavior disorder.” Trends Neurosci 37(5):279–88 [DOI] [PubMed] [Google Scholar]
- Petrova M, Pavlova R, Zhelev Y, Mehrabian S, Raycheva M and Traykov L (2016). “Investigation of neuropsychological characteristics of very mild and mild dementia with Lewy bodies.” J Clin Exp Neuropsychol 38(3): 354–360. [DOI] [PubMed] [Google Scholar]
- Piette T, Mescola P, Uytdenhoef P, Henriet M, Vanderkelen B, Jacquy J, et al. (2007). “A unique episode of REM sleep behavior disorder triggered during surgery for Parkinson’s disease.” J Neurol Sci 253(1–2): 73–76. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Cortelli P, Montagna P, De Monte A, Corsini R, Contin M, et al. (1998). “REM sleep behaviour disorder differentiates pure autonomic failure from multiple system atrophy with autonomic failure.” J Neurol Neurosurg Psychiatry 64(5): 683–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plazzi G and Montagna P (2002). “Remitting REM sleep behavior disorder as the initial sign of multiple sclerosis.” Sleep Med 3(5): 437–439. [DOI] [PubMed] [Google Scholar]
- Plazzi G, Montagna P, Meletti S and Lugaresi E (2002). “Polysomnographic study of sleeplessness and oneiricisms in the alcohol withdrawal syndrome.” Sleep Med 3(3): 279–282. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Arnulf I, Hogl B, Iranzo A, Miyamoto T, Dauvilliers Y, et al. (2012). “A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study.” Mov Disord 27(7): 913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Bertrand JA, Genier Marchand D and Montplaisir JY (2015). “Parkinson risk in idiopathic REM sleep behavior disorder: preparing for neuroprotective trials.” Neurology 84(11): 1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF and Montplaisir JY (2013). “REM Sleep Behavior Disorder and Prodromal Neurodegeneration - Where Are We Headed?” Tremor Other Hyperkinet Mov (N Y) 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Tuineaig M, Bertrand JA, Latreille V, Desjardins C, et al. (2013). “Antidepressants and REM sleep behavior disorder: isolated side effect or neurodegenerative signal?” Sleep 36(11): 1579–1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M, Desjardins C and Montplaisir JY (2011). “Olfaction and color vision identify impending neurodegeneration in rapid eye movement sleep behavior disorder.” Ann Neurol 69(5): 811–818. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M and Montplaisir JY (2009). “Idiopathic REM sleep behavior disorder in the transition to degenerative disease.” Mov Disord 24(15): 2225–2232. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Gagnon JF, Vendette M and Montplaisir JY (2009). “Markers of neurodegeneration in idiopathic rapid eye movement sleep behaviour disorder and Parkinson’s disease.” Brain 132(Pt 12): 3298–3307. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Iranzo A, Hogl B, Arnulf I, Ferini-Strambi L, Manni R, et al. (2015). “Risk factors for neurodegeneration in idiopathic rapid eye movement sleep behavior disorder: a multicenter study.” Ann Neurol 77(5): 830–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postuma RB, Lanfranchi PA, Blais H, Gagnon JF and Montplaisir JY (2010). “Cardiac autonomic dysfunction in idiopathic REM sleep behavior disorder.” Mov Disord 25(14): 2304–2310. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Lang AE, Gagnon JF, Pelletier A and Montplaisir JY (2012). “How does parkinsonism start? Prodromal parkinsonism motor changes in idiopathic REM sleep behaviour disorder.” Brain 135(Pt 6): 1860–1870. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Lang AE, Massicotte-Marquez J and Montplaisir J (2006). “Potential early markers of Parkinson disease in idiopathic REM sleep behavior disorder.” Neurology 66(6): 845–851. [DOI] [PubMed] [Google Scholar]
- Postuma RB, Montplaisir JY, Pelletier A, Dauvilliers Y, Oertel W, Iranzo A, et al. (2012). “Environmental risk factors for REM sleep behavior disorder: a multicenter case-control study.” Neurology 79(5): 428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provini F, Vetrugno R, Pastorelli F, Lombardi C, Plazzi G, Marliani AF, et al. (2004). “Status dissociatus after surgery for tegmental ponto-mesencephalic cavernoma: a state-dependent disorder of motor control during sleep.” Mov Disord 19(6): 719–723. [DOI] [PubMed] [Google Scholar]
- Rahayel S, Montplaisir J, Monchi O, Bedetti C, Postuma RB, Brambati S, et al. (2015). “Patterns of cortical thinning in idiopathic rapid eye movement sleep behavior disorder.” Mov Disord 30(5): 680–687. [DOI] [PubMed] [Google Scholar]
- Rahmani F, Ansari M, Pooyan A, Mirbagheri MM and Aarabi MH (2016). “Differences in white matter microstructure between Parkinson’s disease patients with and without REM sleep behavior disorder.” Conf Proc IEEE Eng Med Biol Soc 2016: 1124–1126. [DOI] [PubMed] [Google Scholar]
- Ramos-Campoy O, Gaig C, Villas M, Iranzo A and Santamaria J (2017). “REM sleep behavior disorder causing subdural hematoma.” Sleep Med 30: 43–44. [DOI] [PubMed] [Google Scholar]
- Rodrigues Brazete J, Montplaisir J, Petit D, Postuma RB, Bertrand JA, Genier Marchand D, et al. (2013). “Electroencephalogram slowing in rapid eye movement sleep behavior disorder is associated with mild cognitive impairment.” Sleep Med 14(11): 1059–1063. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Boeve BF and Mahowald MW (2013). “Delayed emergence of a parkinsonian disorder or dementia in 81% of older men initially diagnosed with idiopathic rapid eye movement sleep behavior disorder: a 16-year update on a previously reported series.” Sleep Med 14(8): 744–748. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR, Ettinger MG and Mahowald MW (1986). “Chronic behavioral disorders of human REM sleep: a new category of parasomnia.” Sleep 9(2): 293–308. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Bundlie SR and Mahowald MW (1996). “Delayed emergence of a parkinsonian disorder in 38% of 29 older men initially diagnosed with idiopathic rapid eye movement sleep behaviour disorder.” Neurology 46(2): 388–393. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Garcia-Rill E, Segall M, Noreen H and Mahowald MW (1996). “HLA class II genes associated with REM sleep behavior disorder.” Ann Neurol 39(2): 261–263. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Lee SA, Bornemann MA and Mahowald MW (2009). “Potentially lethal behaviors associated with rapid eye movement sleep behavior disorder: review of the literature and forensic implications.” J Forensic Sci 54(6): 1475–1484. [DOI] [PubMed] [Google Scholar]
- Schenck CH and Mahowald MW (1992). “Motor dyscontrol in narcolepsy: rapid-eye-movement (REM) sleep without atonia and REM sleep behavior disorder.” Ann Neurol 32(1): 3–10. [DOI] [PubMed] [Google Scholar]
- Schenck CH and Mahowald MW (2002). “REM sleep behavior disorder: clinical, developmental, and neuroscience perspectives 16 years after its formal identification in SLEEP.” Sleep 25(2): 120–138. [DOI] [PubMed] [Google Scholar]
- Schenck CH, Mahowald MW, Kim SW, O’Connor KA and Hurwitz TD (1992). “Prominent eye movements during NREM sleep and REM sleep behavior disorder associated with fluoxetine treatment of depression and obsessive-compulsive disorder.” Sleep 15(3): 226–235. [DOI] [PubMed] [Google Scholar]
- Schenkel E and Siegel JM (1989). “REM sleep without atonia after lesions of the medial medulla.” Neurosci Lett 98(2): 159–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherfler C, Frauscher B, Schocke M, Iranzo A, Gschliesser V, Seppi K, et al. (2011). “White and gray matter abnormalities in idiopathic rapid eye movement sleep behavior disorder: a diffusion-tensor imaging and voxel-based morphometry study.” Ann Neurol 69(2): 400–407. [DOI] [PubMed] [Google Scholar]
- Schrempf W, Katona I, Dogan I, Felbert VV, Wienecke M, Heller J, et al. (2016). “Reduced intraepidermal nerve fiber density in patients with REM sleep behavior disorder.” Parkinsonism Relat Disord 29: 10–16. [DOI] [PubMed] [Google Scholar]
- Siegel JM, Nienhuis R, Fahringer HM, Paul R, Shiromani P, Dement WC, et al. (1991). “Neuronal activity in narcolepsy: identification of cataplexy-related cells in the medial medulla.” Science 252(5010): 1315–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sixel-Doring F, Trautmann E, Mollenhauer B and Trenkwalder C (2011). “Associated factors for REM sleep behavior disorder in Parkinson disease.” Neurology 77(11): 1048–1054. [DOI] [PubMed] [Google Scholar]
- Soja PJ, Lopez-Rodriguez F, Morales FR and Chase MH (1995). “Effects of excitatory amino acid antagonists on the phasic depolarizing events that occur in lumbar motoneurons during REM periods of active sleep.” J Neurosci 15(5 Pt 2): 4068–4076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprenger FS, Stefanova N, Gelpi E, Seppi K, Navarro-Otano J, Offner F, et al. (2015). “Enteric nervous system alpha-synuclein immunoreactivity in idiopathic REM sleep behavior disorder.” Neurology 85(20): 1761–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokholm MG, Iranzo A, Ostergaard K, Serradell M, Otto M, Svendsen KB, et al. (2017). “Assessment of neuroinflammation in patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study.” Lancet Neurol 16(10): 789–796. [DOI] [PubMed] [Google Scholar]
- Terzaghi M, Zucchella C, Rustioni V, Sinforiani E and Manni R (2013). “Cognitive performances and mild cognitive impairment in idiopathic rapid eye movement sleep behavior disorder: results of a longitudinal follow-up study.” Sleep 36(10): 1527–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tippmann-Peikert M, Boeve BF and Keegan BM (2006). “REM sleep behavior disorder initiated by acute brainstem multiple sclerosis.” Neurology 66(8): 1277–1279. [DOI] [PubMed] [Google Scholar]
- Tolosa E and Pont-Sunyer C (2011). “Progress in defining the premotor phase of Parkinson’s disease.” J Neurol Sci 310(1–2): 4–8. [DOI] [PubMed] [Google Scholar]
- Unger MM, Belke M, Menzler K, Heverhagen JT, Keil B, Stiasny-Kolster K, et al. (2010). “Diffusion tensor imaging in idiopathic REM sleep behavior disorder reveals microstructural changes in the brainstem, substantia nigra, olfactory region, and other brain regions.” Sleep 33(6): 767–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendette M, Montplaisir J, Gosselin N, Soucy JP, Postuma RB, Dang-Vu TT, et al. (2012). “Brain perfusion anomalies in rapid eye movement sleep behavior disorder with mild cognitive impairment.” Mov Disord 27(10): 1255–1261. [DOI] [PubMed] [Google Scholar]
- Vetrivelan R, Fuller PM, Tong Q and Lu J (2009). “Medullary circuitry regulating rapid eye movement sleep and motor atonia.” J Neurosci 29(29): 9361–9369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilas D, Iranzo A, Pont-Sunyer C, Serradell M, Gaig C, Santamaria J et al. (2015). “Brainstem raphe and substantia nigra echogenicity in idiopathic REM sleep behavior disorder with comorbid depression.” J Neurol 262(7): 1665–1672. [DOI] [PubMed] [Google Scholar]
- Vilas D, Iranzo A, Tolosa E, Aldecoa I, Berenguer J, Vilaseca I, et al. (2016). “Assessment of alpha-synuclein in submandibular glands of patients with idiopathic rapid-eye-movement sleep behaviour disorder: a case-control study.” Lancet Neurol 15(7): 708–718. [DOI] [PubMed] [Google Scholar]
- Visanji N and Marras C (2015). “The relevance of pre-motor symptoms in Parkinson’s disease.” Expert Rev Neurother 15(10): 1205–1217. [DOI] [PubMed] [Google Scholar]
- Wing YK, Li SX, Mok V, Lam SP, Tsoh J, Chan A, et al. (2012). “Prospective outcome of rapid eye movement sleep behaviour disorder: psychiatric disorders as a potential early marker of Parkinson’s disease.” J Neurol Neurosurg Psychiatry 83(4): 470–472. [DOI] [PubMed] [Google Scholar]
- Winkelman JW and James L (2004). “Serotonergic antidepressants are associated with REM sleep without atonia.” Sleep 27(2): 317–321. [DOI] [PubMed] [Google Scholar]
- Xi Z and Luning W (2009). “REM sleep behavior disorder in a patient with pontine stroke.” Sleep Med 10(1): 143–146. [DOI] [PubMed] [Google Scholar]
- Yeh SB, Yeh PY and Schenck CH (2010). “Rivastigmine-induced REM sleep behavior disorder (RBD) in a 88-year-old man with Alzheimer’s disease.” J Clin Sleep Med 6(2): 192–195. [PMC free article] [PubMed] [Google Scholar]
- Youn S, Kim T, Yoon IY, Jeong J, Kim HY, Han JW, et al. (2016). “Progression of cognitive impairments in idiopathic REM sleep behaviour disorder.” J Neurol Neurosurg Psychiatry 87(8): 890–896. [DOI] [PubMed] [Google Scholar]
- Zagrodzka J, Hedberg CE, Mann GL and Morrison AR (1998). “Contrasting expressions of aggressive behavior released by lesions of the central nucleus of the amygdala during wakefulness and rapid eye movement sleep without atonia in cats.” Behav Neurosci 112(3): 589–602. [DOI] [PubMed] [Google Scholar]
- Zambelis T, Paparrigopoulos T and Soldatos CR (2002). “REM sleep behaviour disorder associated with a neurinoma of the left pontocerebellar angle.” J Neurol Neurosurg Psychiatry 72(6): 821–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Hao Y, Jia F, Tang Y, Li X, Liu W, et al. (2013). “Sertraline and rapid eye movement sleep without atonia: an 8-week, open-label study of depressed patients.” Prog Neuropsychopharmacol Biol Psychiatry 47: 85–92. [DOI] [PubMed] [Google Scholar]