Figure 3.
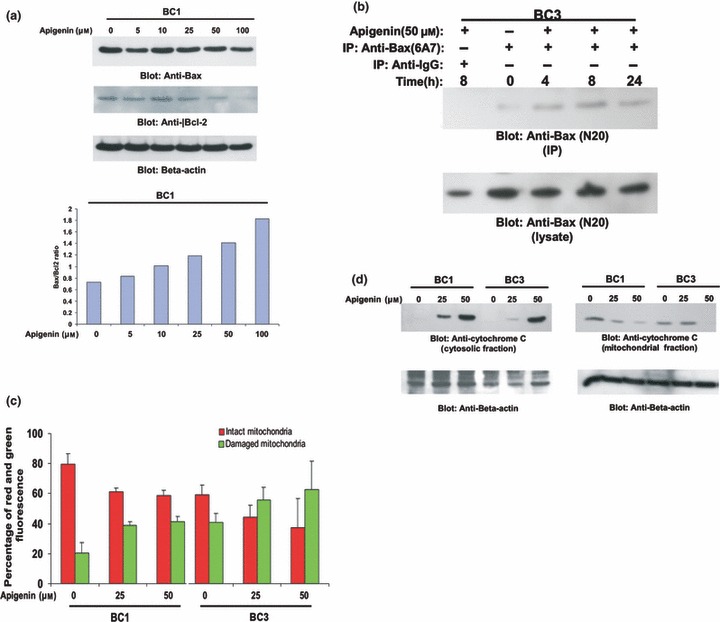
Apigenin‐induced mitochondrial signalling pathways in primary effusion lymphoma cells. (a) Apigenin treatment causes alteration in Bcl‐2 expression. BC1 cells were treated with various doses of apigenin. Cells were lysed and equal amounts of proteins were separated by SDS–PAGE, transferred to Immobilon membrane, and immunoblotted with antibodies against Bax, Bcl‐2 and beta actin as indicated (upper panel). Data obtained from immunoblot analyses of Bax and Bcl‐2 were used to evaluate effects of apigenin on Bax/Bcl‐2 ratio. Densitometric analysis of Bax and Bcl‐2 bands was performed using AlphaImager Software (San Leandro, CA, USA), and data (relative density normalized to β‐actin) were plotted as Bax/Bcl‐2 ratio. (b) Apigenin‐induced Bax activation. After treating with 50 μm apigenin for indicated time periods, BC1 and BC3 cells were lysed in 1% Chaps lysis buffer and subjected to immunoprecipitation with either anti‐Bax 6A7 antibody or non‐specific IgG, for detection of conformationally changed Bax protein. In addition, total cell lysates were applied directly to SDS–PAGE, transferred to Immobilon membrane and immunoblotted with specific anti‐Bax polyclonal antibody. (c) Loss of mitochondrial potential by apigenin treatment. Cells were treated with and without 25 and 50 μm apigenin for 24 h. Live cells with intact mitochondrial membrane potential (red bars) and dead cells with lost mitochondrial membrane potential (green bars) were measured by JC‐1 staining and analysed by flow cytometry as described in the Materials and methods section. Average of three independent experiments is depicted. (d) Apigenin‐induced release of cytochrome c. BC1 and BC3 cells were treated with and without 25 and 50 μm apigenin for 24 h. Mitochondrial‐free cytoplasmic fractions as well as mitochondrial extracts were isolated as described in the Materials and Methods sections. Cell extracts were separated on SDS–PAGE, transferred to PVDF membrane, and immunoblotted with an antibody against cytochrome c. Beta‐actin was used for equal loading.
