Figure 6.
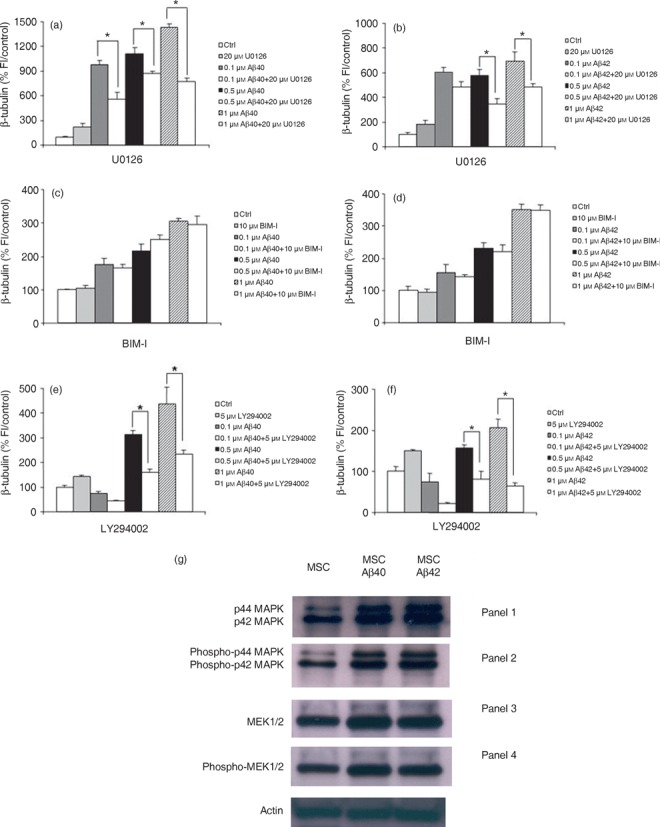
Neuronal differentiation induced by amyloid beta (Aβ) is mediated by ERK1/2 via upstream activation of the PI(3)K‐dependent pathway. Bone marrow‐derived mesenchymal stem cells (BM‐MSC) were treated with aggregated Aβs in the presence or absence of U0126, bisindolylmaleimide I or LY294002. (a,b) MEK blockade with U0126 abolishes Aβ‐induced neuronal differentiation as determined by a decrease in expression of the neuronal marker β‐tubulin. (c,d) Aβs do not induce neuronal differentiation through the protein kinase C pathway. Co‐incubation of cultures for 5 days with Aβ peptides in the presence of the highly specific protein kinase C inhibitor BIM‐1 has no effect on the Aβ‐induced increase in neuronal number. (e,f) PI(3)K mediates the Aβ effect. Co‐incubation of cells for 5 days with Aβs in the presence of 5 µm LY294002 which specifically blocks PI(3)K completely abolished the ability of Aβ to induce neuronal differentiation. The graphs show one representative experiment of between three and four different experiments done in quadruplicate. *P < 0.0001, relative to control. (g) Representative Western blot analysis to examine activation of enzymes in the ERK1/2 signalling pathway using antibodies to endogenous non‐phosphorylated and phosphorylated forms of ERK1/2 (MAP kinase) and MEK1/2. (Panel 1) p44/42 MAP Kinase antibody detects endogenous levels of total ERK1/2 (MAP kinase) protein and shows an increase in expression in treated cells compared to controls. (Panel 2) Lanes 2 and 3 demonstrate that there is an increase in phosphorylated forms of p42/44 MAPK compared to untreated cells with addition of Aβ peptide as detected by phospho‐42/44 MAP kinase antibody. This antibody detects endogenous levels of p42 and p44 ERK1/2 (MAP kinase) when phosphorylated at threonine and tyrosine residues. (Panel 3) Endogenous MEK1/2 detected by the MEK1/2 antibody (Panel 4) confirms an increase in phosphorylated enzyme, phospho‐MEK1/2 compared to untreated cells in response to Aβ treatment.
