Abstract
Objectives: To explore the role of Oct3/4, Nanog and Sox2 in regeneration of rat tracheal epithelium.
Materials and methods: An ex vivo model of rat tracheal epithelial regeneration using 5‐fluorouracil (5‐FU) was developed, to induce injury. Expression levels of Oct3/4, Nanog and Sox2 were examined using Western blot analysis, RT‐PCR or microscopically observed immunofluorescence, and cell morphological changes were observed using HE staining, during the recovery process.
Results: Oct3/4, Nanog and Sox2 were not detectable in normal tracheal epithelium. After treatment with 5‐FU, the normally proliferating tracheal epithelium desquamated and only a few cells in G0 phase of the cell cycle were left on the basement membrane and Oct3/4, Nanog and Sox2 could be observed at this time. Thereafter, the number of Oct3/4‐, Nanog‐ and Sox2‐positive cells increased gradually. When the cells differentiated into ciliate cells, mucous cells or basal cells, and restored pseudostratified mucociliary epithelium, the number of Oct3/4‐, Nanog‐ and Sox2‐positive cells decreased and gradually disappeared.
Conclusions: G0 phase cells with resistance to 5‐FU damage expressed Oct3/4, Nanog and Sox2. This indicated that these cells were undifferentiated, but had the ability to terminally differentiate into downstream‐type cells. They possessed stem cell properties. The results are consistent with Oct3/4, Nanog and Sox2‐expressing cells being considered as tracheal stem cells.
Introduction
A concept of a correlative genome for embryonic stem (ES) cells has been proposed, recently, which is expressed only in the ES cells, but not in mature somatic cells. This plays an important role in maintaining the undifferentiated state and pluripotency of stem cells. Oct3/4, Nanog and Sox2 genes are typical examples of this genome. Oct3/4 is a synomyn for Pou5f1 which encodes Pou5fl protein, a member of the POU family of transcription factors (1, 2, 3). A marker of totipotency, it can promote formation of inner cell mass (ICM) and maintain the undifferentiated state of ES cells (4, 5, 6). Oct3/4 can be expressed in many kinds of totipotent cells including oocytes, archaeocytes, pre‐implantation embryos, primitive ectoderm, ICM and ES cells (7, 8, 9, 10). However, it is never expressed in differentiated daughter cells (11, 12, 13).
Recently, several co‐factors of Oct3/4 have been identified including ELA, Sox2 (Sry‐related factor) and Rox‐1 (14, 15). ELA was thought to be a bridging factor between Oct3/4 and the basic transcription machinery and Sox2 can activate Utf1 and Fgf‐4 genes (16, 17). These target genes of Oct3/4 are expressed in different embryonic tissues and regulating expression of these genes maintains normal development of embryos (5, 18). Nanog is a newly identified transcriptional factor and evidence has shown that it is expressed specifically in ICM, ES cells and EG cells (19, 20), but not in differentiated, mature somatic cells (21, 22). Nanog‐deficient ES cells lose their pluripotency and begin to differentiate, as Nanog is an important regulator for maintaining pluripotent and self‐renewal capacities of ES cells (23). Remarkably, these pluripotency factors, Oct3/4, Sox2 and Nanog, have also been shown to participate in reprogramming of differentiated cells back to the pluripotential state (24, 25).
Recent studies have shown that Oct3/4 is expressed in a variety of somatic stem cells and in multipotent somatic progenitor cells, where it has been suggested that it functions in a manner analogous to its role in ES cells (26, 27, 28); Sox2 and Nanog may regulate expression of Oct3/4 (29). An ex vivo model of rat tracheal epithelial regeneration using 5‐fluorouracil (5‐FU) was developed to induce tissue injury (30). In the trachea, there are tracheal stem cells which when in the G0 phase have resistance to 5‐FU. To identify new markers of tracheal stem cells, the same model was used in the present study and expression of Oct3/4, Nanog and Sox2 detected to study their roles in this tissue.
Materials and Methods
Model for tracheal epithelial regeneration
Male and female Wistar rats (∼200 g) were used in accordance with the guidelines of the Animal Care Committee of the China Medical University. Material and reagents for tissue culture were purchased from Invitrogen (Carlsbad, CA, USA). Animals were killed and tracheas were excised under sterile conditions and then cultured in 1:1 mixture of Dulbecco’s Modified Eagle’s Medium and Ham’s F‐12 medium (DMEM/F12) containing 120 mg/mL 5‐FU and 10% foetal bovine serum (FBS), for 12 h at 37 °C. After washing off the 5‐FU, tracheas were continued to be cultured in DMEM/F12 containing 10% FBS. After 0, 3, 6, 12, 24 and 48 h in culture, the tracheas were collected. For RT‐PCR and Western blot analyses, tracheal epithelia was digested and stored at −80 °C until further use. For immunofluorescence analysis, tracheas were fixed in 4% paraformaldehyde and prepared for paraffin‐embedded tissue sections (3 μm thick) with haematoxylin–eosin (HE) staining and immunofluorescence investigation.
Isolation of tracheal epithelial cells
Epithelial cells were isolated from treated and untreated tracheas. Tracheas were digested by filling lumina of ligated trachea with 1 mg/mL proteinase XIV solution (Sigma‐Aldrich, St Louis, MO, USA) in DMEM/F12 without serum, at 4 °C overnight. Enzymatic digestion was terminated by adding 10 mL of DMEM/F12 containing 10% FBS. Cell aggregates were collected, a subset was stored at −80 °C for further analysis using RT‐PCR and Western blotting, while other fractions were pooled and centrifuged at 1500 g for 5 min at room temperature. After removal of supernatants, pellets were resuspended and washed in 10 mL of phosphate‐buffered saline (PBS), centrifuged at 1500 g for 5 min and resuspended in 0.5 mL of PBS. Cells were labelled while in the PBS with 5.0 μg/mL Hoechst33342 dye, the method being basically as described previously (31).
Indirect immunofluorescence
Indirect immunofluorescence staining was performed using Oct3/4, Nanog and Sox2 antibodies, on serial tissue sections (3 μm thickness) of tracheas during the recovery from injury period (Santa Cruz Biotechnology, Santa Cruz, CA, USA), using an experimental protocol as described previously (30). Briefly, goat anti‐Oct3/4, rabbit anti‐Nanog and goat anti‐Sox2 (dilution 1:100) were used as primary antibodies. Rhodamine isothiocyanate (TRITC)‐conjugated rabbit antigoat IgG and fluorescein isothiocyanate‐conjugated goat anti‐rabbit immunoglobulin G (IgG) (dilution 1:100; HuaMei, Beijing, China) were used as secondary antibodies, which were diluted with 1% bovine serum albumin (BSA)–PBS. After treatment with the secondary antibody, specimens were incubated with 0.5 μg/mL of 4, 6‐diamidino‐2‐phenylindole (DAPI; Sigma) for nuclear counterstaining. Specimens were examined using an epi‐illumination fluorescence microscope BX50 (Olympus, Tokyo, Japan). For serum controls, 1% BSA–PBS instead of the primary antibody was used as a negative control.
Western blotting and densitometric analyses
Total cell homogenates were prepared by lysing cells in NP40 lysis buffer consisting of 1% NP40, 10% glycerol, 20 mm Tris–HCl pH 8.0, 137 mm NaCl and 4% complete protease inhibitor cocktail mix (Roche, Mannheim, Germany). Eighty micrograms of total protein was used for sodium dodecyl sulphate–polyacrylamide gel electrophoresis, followed by transfering blotting to Polyvinylidene fluoride (PVDF) (Immobilon; Millipore Corp., Billerica, MA, USA). Membranes were blocked with 5% non‐fat dried milk in PBS for 1 h with gentle shaking, and were then washed three times, 10 min each wash, with PBS containing 0.1% Tween 20. Membranes were incubated with primary antibody (Table 1, all antibodies from Santa Cruz) in 1% BSA in PBS overnight at 4 °C with shaking. Membranes were washed as described earlier and incubated with secondary antibodies (Table 1) for 2 h at room temperature. They were then washed again and incubated with 3,3′‐diaminobenzidine (DAB) for 2–3 min at room temperature. When bands reached the desired darkness (2–5 min), membranes were washed briefly in water followed with PBS. Finally, membranes were dried and photographed. After scanning, densitometric analysis was performed using ImageJ 1.33 software (National Institutes of Health, Bethesda, MD, USA).
Table 1.
Antibodies used in Western blot analysis
| Primary antibody | Secondary antibody (peroxidase‐conjugated) | |||||
|---|---|---|---|---|---|---|
| Name | Source | Dilution | Product no. | Name | Dilution | Product no. |
| Oct3/4 | Goat | 1:200 | sc‐8628 | Rabbit antigoat IgG | 1:2000 | sc‐2768 |
| Sox2 | 1:100 | sc‐17320 | ||||
| Nanog | Rabbit | 1:100 | sc‐33760 | Goat antirabbit IgG | 1:2000 | sc‐2004 |
| β‐actin | 1:200 | sc‐7210 | ||||
RT‐PCR analysis
Total RNA was extracted from cells harvested using TRIzol reagent (Invitrogen). RT‐PCR was performed with the TaKaRa RNA PCR Kit (AMV) version 3.0 (Takara Bio, Otsu, Japan) according to the manufacturer’s protocol. PCR primers were designed span exons, to exclude the possibility of genomic contamination (Table 2), and β‐actin was used as internal control. PCR conditions were as follows: 94 °C for 2 min, 94 °C for 30 s, variable temperature (see Table 2) for 40 s, and 72 °C for 1 min, for 35 cycles. Reverse transcription reactions lacking reverse transcriptase served as negative controls. PCR products were visualized by ethidium bromide staining on 2% agarose gels using a gel scanner (UVP, Cambridge, UK).
Table 2.
RT‐PCR primer sequences and product sizes
| Gene | Primer sequence (5′→3′) | Accession number | Size (bp) | Annealing temp. (°C) |
|---|---|---|---|---|
| Oct3/4 | agg cag gag cac gag tgg a cga agc ggc aga tgg ttg t | NM_001009178 | 264 | 58.3 |
| Nanog | tct cct ccg cct tcc tct ttg cct ctg aaa cct atc ctt g | NM_001100781 | 204 | 53.1 |
| Sox2 | ggg ctc tgt ggt caa gtc tag tcg gca tca cgg ttt | NM_001109181 | 435 | 62.1 |
| β‐actin | cca agg cca acc gcg aga aga tga c agg gta cat ggt ggt gcc gcc aga c | NM_031144 | 587 | 58 |
Statistical analysis
Data from at least three independent experiments were used for statistical analysis by the spss 11.5 (SPSS Inc., Chicago, IL, USA). All values were expressed as mean ± SD. Statistical analyses were performed using one‐way anova, where P < 0.05 was considered significant.
Results
Morphological changes in rat tracheal epithelium after ex vivo injury induced by 5‐FU
Normal rat trachea and rat tracheas treated with 5‐FU were analysed using HE staining, to investigate morphological changes in rat tracheal epithelium (Fig. 1). Immediately after 5‐FU treatment (0 h), only a few cells with less cytoplasm were observed on the basement membrane. Three hours later, cytoplasm extended along basement membranes and cells appeared flattened. After 6 h, the number of flattened cells increased and they appeared to become cuboidal by 12 h. The number of flattened cells increased sharply at 24 h. At roughly 48 h, cilia were observed on the surface of the tracheal epithelium, which appeared pseudostratified and almost completely normal.
Figure 1.
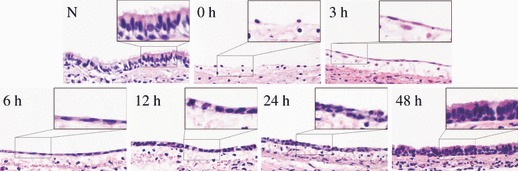
Morphological changes in rat tracheal epithelium after injury induced by 5‐FU as observed by HE staining. N) Normal rat tracheal epithelium is pseudostratified. 0 h) Only a few cells with less cytoplasm than normal tracheal epithelial cells remained on the basement membrane immediately after 5‐FU treatment. 3 h) Cytoplasm of these cells extended along the basement membrane and cells became to look flat. 6 h) The number of flat cells increased and they had less cytoplasm 12 h) Cells appeared cuboidal. 24 h) The number of cells increased sharply. 48 h) Cilia appeared on the luminal side of the trachea, tracheal epithelium became pseudostratified and appeared to be almost completely recovered (original magnification ×400)
Expression of Oct3/4, Nanog and Sox2 in tracheal epithelium as detected by indirect immunofluorescence
To examine localization of Oct3/4, Nanog and Sox2, immunofluorescence staining was performed on serial sections of the tracheal epithelium with and without 5‐FU treatment. Bright green fluorescence in cells indicated Nanog positive expression, and bright red fluorescence indicated Oct3/4 and Sox2 positive expression.
It was observed that Oct3/4 was negative in normal tracheal epithelium. Immediately after 5‐FU treatment, very few Oct3/4‐positive cells were detected. Subsequently, the number of Oct3/4‐positive cells increased gradually and reached the maximal level at around 6 h (Table 3). Then its expression began to decrease and returned to baseline levels after 48 h. Expression patterns of Nanog and Sox2 were similar to those of Oct3/4, and all three were all localized in the same cells (Fig. 2).
Table 3.
Cell number of different cell types in tracheal epithelium per five fields (×400) during regeneration
| Time | Type | N | 0 h | 3 h | 6 h | 12 h | 24 h | 48 h |
|---|---|---|---|---|---|---|---|---|
| Oct3/4(+) | Number | 0 | 10 | 19 | 63 | 31 | 12 | 5 |
| Ratio* | 0 | 0.400 | 0.475 | 0.830 | 0.221 | 0.070 | 0.020 | |
| Nanog(+) | Number | 0 | 10 | 21 | 65 | 30 | 10 | 4 |
| Ratio* | 0 | 0.400 | 0.525 | 0.867 | 0.214 | 0.059 | 0.016 | |
| Sox2(+) | Number | 0 | 9 | 18 | 62 | 33 | 10 | 5 |
| Ratio* | 0 | 0.360 | 0.450 | 0.827 | 0.236 | 0.059 | 0.020 | |
| Total | 252 | 25 | 40 | 75 | 140 | 170 | 249 |
*Ratio = Cell number of different cell types/total cell number.
Figure 2.
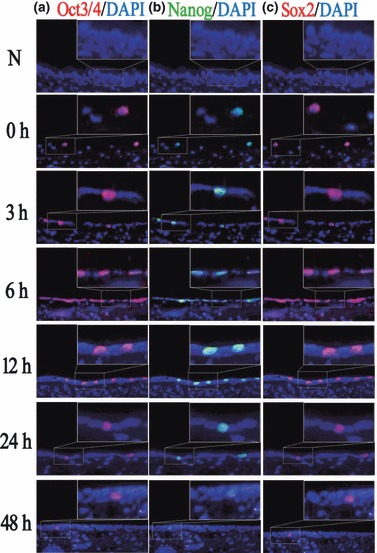
Immunofluorescence staining of Oct3/4, Nanog and Sox2 during recovery from injury induced by 5‐FU. (a) In untreated rat tracheal epithelium, Oct3/4 was not expressed. After treatment with 5‐FU, the number of Oct3/4‐positive cells increased gradually, reaching its peak at about 6 h, then decreased gradually and returned to its normal levels at about 48 h. (b,c) Expression tendency of Nanog and Sox2 was similar to that of Oct3/4, as they all are located in the same cells (original magnification ×400).
Expression of Oct3/4, Nanog and Sox2 in tracheal epithelium as detected by Western blot analysis
To confirm further changes in Oct3/4, Nanog and Sox2 during regeneration of tracheal epithelium, the protein levels of Oct3/4, Nanog and Sox2 (Fig. 3a) were examined in after 5‐FU treatment, using Western blot analysis. Results showed similar expression trends for all Oct3/4, Nanog and Sox2. However, Oct3/4, Nanog and Sox2 were not detectable in normal rat tracheal epithelium. After treatment with 5‐FU, expression levels increased and reached the maximal level at around 6 h, and decreased gradually to return to very low levels by approximately 48 h (Fig. 3b). This indicated that changes in Oct3/4, Nanog and Sox2 levels were consistent with results obtained by immunofluorescence staining.
Figure 3.
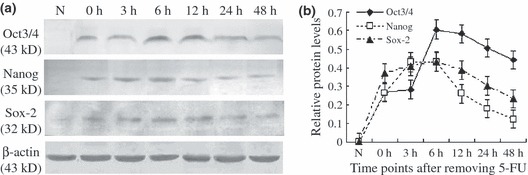
Expression levels of Oct3/4, Nanog and Sox2 proteins in rat tracheal epithelium during recovery from 5‐FU induced injury. (a) Western blot analysis of Oct3/4, Nanog and Sox2 proteins in normal trachea and 5‐FU‐treated tracheas. (b) Expression levels of Oct3/4, Nanog and Sox2 relative to β‐actin. Data are presented as mean ± SD of three independent experiments.
Expression levels of Oct3/4, Nanog and Sox2 in tracheal epithelium as detected by RT‐PCR analysis
Change in trend of Oct3/4, Nanog and Sox2 levels is in accordance with results obtained by Western blotting (Fig. 4a). There was no detectable Oct3/4, Nanog and Sox2 mRNA in normal tracheal epithelium. After treatment with 5‐FU, expression levels increased and reached peak levels at about 6 h, and decreased gradually thereafter to return to very low levels by about 48 h (Fig. 4b).
Figure 4.
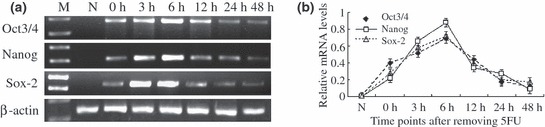
Expression of Oct3/4, Nanog and Sox2 in the rat tracheal epithelium. (a) mRNA levels of Oct3/4, Nanog and Sox2 in the rat tracheal epithelium before and after 5‐FU treatment, were examined using RT‐PCR. (b) Expression levels of Oct3/4, Nanog and Sox2 relative to β‐actin. Data are presented as mean ± SD of three independent experiments.
Hoechst33342 labelling in the tracheal epithelium cells
To detect tracheal stem cells existing in the epithelium after treatment with 5‐FU, we stained them with Hoechst33342 dye, which has been proved to provide dull staining in stem cells (32). As shown in Fig. 5, cells with no staining with Hoechst33342 under UV excitation, but with clear round cellular morphology under the bright visual field might be tracheal stem cells.
Figure 5.
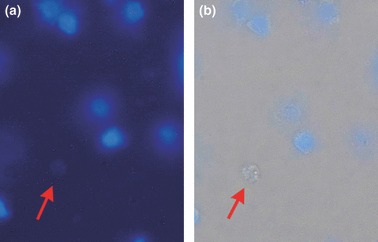
Tracheal epithelial cell smears with Hoechst 33342. (a) After UV excitation, blue nuclei could be seen; (b) cell shapes under bright field visual illumination compared with the same cells with no Hoechst33342 dye combination (arrow).
Discussion
Borthwick et al. (33) have shown that bromodeoxyuridine (BrdUrd) label‐retaining cells (LRC) represent the tracheal stem cell compartment, which are localized to glands/ducts in the upper trachea and to systematically arrayed foci in the lower trachea, where the BrdUrd LRC labelling index exceeded 30%. However, Kiel et al. (34) confirmed that less than 6% of haematopoietic stem cells (HSCs) retain BrdUrd and less than 0.5% of all BrdUrd‐retaining haematopoietic cells were HSCs, revealing that BrdUrd has poor specificity and poor sensitivity as an HSC marker. Therefore reliability of BrdUrd label‐retaining tracheal stem cells remains to be elucidated. Recent reports have indicated that more than 95% HSCs (35, 36) or epidermal stem cells (37) are in the G0 phase of the cell cycle, which provided an opportunity for us to investigate stem cells in G0 phase cells. A regeneration model of ex vivo trachea damaged by 5‐FU has been constructed by us previously (30). 5‐FU is a member of the antimetabolite drug family and after treatment with 5‐FU, normally proliferating tracheal epithelium desquamated, leaving only a few cells in G0 phase at the basement membrane (38, 39, 40).These cells were PCNA negative, indicating that they were in G0. In this study, we have repeatedly adjusted the concentration of 5‐FU to maintain integrity of the basement membrane, this was indicated by silver staining (data not shown). After removal of 5‐FU, the tracheal epithelium became flat then cuboidal, then recovered into pseudostratified epithelium. This process was only achieved by viable G0 cells left on the basement membrane. Our results confirmed that these cells were Hoechst 33342 negative and ABCG2 positive (30). This process also occurred in the in vivo model (41). Our results suggested that these cells might be tracheal stem cells.
The transcriptional regulator Oct3/4 is a Pou domain‐containing protein expressed in pluripotent embryonic cells and cells of the germline, where its inactivation results in loss of pluripotency and apoptosis respectively (42, 43). Oct3/4 interacts with other embryonic regulators, such as Sox2 and Nanog, to oversee a vast regulatory network that maintains pluripotency and inhibits cell differentiation (44, 45). In this study, it was observed that Oct3/4, Nanog and Sox2 were not expressed in normal tracheal epithelium. After treatment with 5‐FU, Oct3/4, Nanog and Sox2 were all expressed in G0 phase cells. When the cells differentiated into ciliated cells, mucous cells or basal cells, Oct3/4‐, Nanog‐ and Sox2‐positive cells decreased and gradually disappeared. Western blotting and RT‐PCR data confirmed our observation. Oct3/4 gene expression in the adult has been reported in stem cells from a variety of tissues including kidney (46), mammary epithelium (47), pancreatic islets (48), mesenchymal stem cells (26, 27, 28) and liver (49). Upon induction of differentiation, expression of Oct3/4 decreased and disappeared. Oct3/4 may play an important role in self‐renewal of somatic stem cells, and Sox2 and Nanog may regulate expression of Oct3/4; our results were consistent with these studies. Therefore, expression of Oct3/4, Nanog and Sox2 may be used as tracheal stem cell markers. Our study has suggested that Oct3/4, Nanog and Sox2 may not only be crucial for maintenance of pluripotency in embryonic cells, but may also play an important role in self‐renewal of tracheal stem cells and in maintaining tissue homeostasis.
Recent reports have shown that Oct3/4, Nanog and Sox2 were expressed in ES cells. After differentiation, expression of Oct3/4, Nanog and Sox2 decreased and disappeared. These changes were regulated by effects of DNA methylation (50), histone acetylation or microRNAs (51). Thus, expression changes of Oct3/4, Nanog and Sox2 in regeneration of rat tracheal epithelium might use mechanisms similar to those of ES cells and further investigation is needed.
Acknowledgements
This study was supported by National Natural Science Foundation of China (grant 30170407 to X. S. Jia).
References
- 1. Okamoto K, Okazawa H, Okuda A, Sakai M, Muramatsu M, Hamada H (1990) A novel octamer binding transcription factor is differentially expressed in mouse embryonic cells. Cell 60, 461–472. [DOI] [PubMed] [Google Scholar]
- 2. Rosner MH, Vigano MA, Ozato K, Timmons PM, Poirier F, Rigby PW et al. (1990) A POU‐domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature 345, 686–692. [DOI] [PubMed] [Google Scholar]
- 3. Schöler HR, Ruppert S, Suzuki N, Chowdhury K, Gruss P (1990) New type of POU domain in germ line‐specific protein Oct‐4. Nature 344,435–439. [DOI] [PubMed] [Google Scholar]
- 4. Palmieri SL, Peter W, Hess H, Schöler HR (1994) Oct‐4 transcription factor is differentially expressed in the mouse embryo during establishment of the first two extraembryonic cell lineages invloved in implantation. Dev. Biol. 166, 259–267. [DOI] [PubMed] [Google Scholar]
- 5. Niwa H (2001) Molecular mechanism to maintain stem cell renewal of ES cells. Cell Struct. Funct. 26, 137–148. [DOI] [PubMed] [Google Scholar]
- 6. Babaie Y, Herwig R, Greber B, Brink TC, Wruck W, Groth D et al. (2007) Analysis of Oct4‐dependent transcriptional networks regulating selfrenewal and pluripotency in human embryonic stem cells. Stem Cells 25, 500–510. [DOI] [PubMed] [Google Scholar]
- 7. SchÖler HR (1991) Octamania: the POU factors in murine development. Trends Genet. 7, 323–329. [DOI] [PubMed] [Google Scholar]
- 8. Pesce M, Gross MK, Scholer HR (1998) In line with our ancestors: Oct‐4 and the mammalian germ. Bioessays 20, 722–732. [DOI] [PubMed] [Google Scholar]
- 9. Hansis C, Grifo JA, Krey LC (2000) Oct‐4 expression in inner cell mass and trophectoderm of human blastocysts. Mol. Hum. Reprod. 6, 999–1004. [DOI] [PubMed] [Google Scholar]
- 10. Pesce M, Scholer HR (2000) Oct‐4: control of totipotency and germline determination. Mol. Reprod. Dev. 55, 452–457. [DOI] [PubMed] [Google Scholar]
- 11. Niwa H, Miyazaki J, Smith G (2000) Quantitative expression of Oct‐3/4 defines differentiation, dedifferentiation or self‐renewal of ES cells. Nat. Genet. 24, 372–376. [DOI] [PubMed] [Google Scholar]
- 12. Solter D (2000) Mammalian cloning: advance and limitations. Nat. Rev. Genet. 1, 199–207. [DOI] [PubMed] [Google Scholar]
- 13. Pesce M, Scholer HR (2000) Oct‐4: gatekeeper in the beginnings of mammalian development. Stem Cells 19, 271–278. [DOI] [PubMed] [Google Scholar]
- 14. Ben‐Shushan E, Thompson JR, Gudas LJ, Bergman Y (1998) Rex‐1, a gene encoding a transcription factor expressed in the early embryo, is regulated via Oct‐3/4 and Oct‐6 binding to an octamer site and a novel protein, Rox‐1, binding to an adjacent site. Mol. Cell. Biol. 18, 1866–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tomioka M, Nishimoto M, Miyagi S, Katayanagi T, Fukui N, Niwa H et al. (2002) Identification of Sox‐2 regulatory region which is under the control of Oct‐3/4–Sox‐2 complex. Nucleic Acids Res. 30, 3202–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Avilion AA, Nicolis SK, Pevny LH, Perez L, Vivian N, Lovell‐Badge R (2003) Multipotent cell lineages in early mouse development depend on SOX2 function. Genes Dev. 17, 126–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Okumura‐Nakanishi S, Saito M, Niwa H, Ishikawa F (2005) Oct‐3/4 and Sox2 regulate Oct‐3/4 gene in embryonic stem cells. J. Biol. Chem. 280, 5307–5317. [DOI] [PubMed] [Google Scholar]
- 18. Wang J, Rao S, Chu J, Shen X, Levasseur DN, Theunissen TW et al. (2006) A protein interaction network for pluripotency of embryonic stem cells. Nature 444, 364–368. [DOI] [PubMed] [Google Scholar]
- 19. Chambers I, Colby D, Robertson M, Nichols J, Lee S, Tweedie S et al. (2003) Functional expression cloning of Nanog, a pluripotency sustaining factor in embryonic stem cells. Cell 113, 643–655. [DOI] [PubMed] [Google Scholar]
- 20. Hart AH, Hartley L, Ibrahim M, Robb L (2004) Identification, cloning and expression analysis of pluripotency promoting Nanog genes in mouse and human. Dev. Dyn. 230, 187–198. [DOI] [PubMed] [Google Scholar]
- 21. Booth HA, Holland PW (2004) Eleven daughters of NANOG. Genomics 84, 229–238. [DOI] [PubMed] [Google Scholar]
- 22. Hatano SY, Tada M, Kimura H, Yamaguchi S, Kono T, Nakano T et al. (2005) Pluripotential competence of cells associated with Nanog activity. Mech. Dev. 122, 67–79. [DOI] [PubMed] [Google Scholar]
- 23. Becskei A, Serrano L (2000) Engineering stability in gene networks by autoregulation. Nature 405, 590–593. [DOI] [PubMed] [Google Scholar]
- 24. Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676. [DOI] [PubMed] [Google Scholar]
- 25. Yu J, Vodyanik MA, Smuga‐Otto K, Antosiewicz‐Bourget J, Frane JL, Tian S et al. (2007) Induced pluripotent stem cell lines derived from human somatic cells. Science 318, 1917–1920. [DOI] [PubMed] [Google Scholar]
- 26. Nayernia K, Lee JH, Drusenheimer N, Nolte J, Wulf G, Dressel R et al. (2006) Derivation of male germ cells from bone marrow stem cells. Lab. Invest. 86, 654–663. [DOI] [PubMed] [Google Scholar]
- 27. Pallante BA, Duignan I, Okin D, Chin A, Bressan MC, Mikawa T et al. (2007) Bone marrow Oct3/4+ cells differentiate into cardiac myocytes via age‐dependent paracrine mechanisms. Circ. Res. 100, e1–e11. [DOI] [PubMed] [Google Scholar]
- 28. Zhang S, Jia Z, Ge J, Gong L, Ma Y, Li T et al. (2005) Purified human bone marrow multipotent mesenchymal stem cells regenerate infarcted myocardium in experimental rats. Cell Transplant. 14, 787–798. [DOI] [PubMed] [Google Scholar]
- 29. Masui S, Nakatake Y, Toyooka Y, Shimosato D, Yagi R, Takahashi K et al. (2007) Pluripotency governed by Sox2 via regulation of Oct3/4 expression in mouse embryonic stem cells. Nat. Cell Biol. 9, 625–635. [DOI] [PubMed] [Google Scholar]
- 30. Ma XB, Jia XS, Liu YL, Wang LL, Sun SL, Song N et al. (2009) Expression and role of notch signaling in the regeneration of rat tracheal epithelium. Cell Prolif. 42, 15–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ding Q, Jia XS (2004) Observation of rat tracheal stem cells during the injure and regeneration induced by 5‐FU. Acta Anat. Sin. 35, 328–330. [Google Scholar]
- 32. Kim M, Turnquist H, Jackson J, Sgagias M, Yan Y, Gong M et al. (2002) The multidrug resistance transporter ABCG2 (breast cancer resistance protein 1) effluxes Hoechst 33342 and is overexpressed in hematopoietic stem cells. Clin. Cancer Res. 8, 22–28. [PubMed] [Google Scholar]
- 33. Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH (2001) Evidence for stem‐cell niches in the tracheal epithelium. Am. J. Respir. Cell Mol. Biol. 24, 649–652. [DOI] [PubMed] [Google Scholar]
- 34. Kiel MJ, He S, Ashkenazi R, Gentry SN, Teta M, Kushner JA et al. (2007) Haematopoietic stem cells do not asymmetrically segregate chromosomes or retain BrdU. Nature 449, 238–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheshier SH, Morrison SJ, Liao X, Weissman IL (1999) In vivo proliferation and cell cycle kinetics of long‐term self‐renewing hematopoietic stem cells. Proc. Natl. Acad. Sci. USA 96, 3120–3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Nygren JM, Bryder D, Jacobsen SE (2006) Prolonged cell cycle transit is a defining and developmentally conserved hemopoietic stem cell property. J. Immunol. 177, 201–208. [DOI] [PubMed] [Google Scholar]
- 37. Dunnwald M, Chinnathambi S, Alexandrunas D, Bickenbach JR (2003) Mouse epidermal stem cells proceed through the cell cycle. J. Cell. Physiol. 195, 194–201. [DOI] [PubMed] [Google Scholar]
- 38. Donowitz GR, Quesenberry P (1986) 5‐Fluorouracil effect on cultured murine stem cell progeny and peripheral leukocytes. Exp. Hematol. 14, 207–214. [PubMed] [Google Scholar]
- 39. Stewart FM, Temeles D, Lowry P, Thraves T, Grosh WW, Quesenberry PJ (1993) Post‐5‐fluorouracil human marrow: stem cell characteristics and renewal properties after autologous marrow transplantation. Blood 81, 2283–2289. [PubMed] [Google Scholar]
- 40. Paiushina OV, Damaratskaia EI, Bueverova EI, Nikonova TM, Butorina NN, Molchanova EA et al. (2006) Analysis of sensitivity of stromal stem cells (CFU‐f) from rat bone marrow and fetal liver to 5‐fluorouracil. Izv. Akad. Nauk Ser. Biol. 6, 660–666. [PubMed] [Google Scholar]
- 41. Liao AJ, Jia XS, Qu Y (2005) The wound‐repair process of rat trachea in vivo and the localization of tracheal stem cells. Chin. J. Histochem. Cytochem. 14, 349–352. [Google Scholar]
- 42. Kehler J, Tolkunova E, Koschorz B, Pesce M, Gentile L, Boiani M et al. (2004) Oct4 is required for primordial germ cell survival. EMBO Rep. 5, 1078–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nichols J, Zevnik B, Anastassiadis K, Niwa H, Klewe‐Nebenius D, Chambers I et al. (1998) Formation of pluripotent stem cells in the mammalian embryo depends on the POU transcription factor Oct 4. Cell 95, 379–391. [DOI] [PubMed] [Google Scholar]
- 44. Boyer LA, Lee TI, Cole MF, Johnstone SE, Levine SS, Zucker JP et al. (2005) Core transcriptional regulatory circuitry in human embryonic stem cells. Cell 122, 947–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loh YH, Wu Q, Chew JL, Vega VB, Zhang W, Chen X et al. (2006) The Oct4 and Nanog transcription network regulates pluripotency in mouse embryonic stem cells. Nat. Genet. 38, 431–440. [DOI] [PubMed] [Google Scholar]
- 46. Gupta S, Verfaillie C, Chmielewski D, Kren S, Eidman K, Connaire J et al. (2006) Isolation and characterization of kidney‐derived stem cells. J. Am. Soc. Nephrol. 17, 3028–3040. [DOI] [PubMed] [Google Scholar]
- 47. Tai MH, Chang CC, Kiupel M, Webster JD, Olson LK, Trosko JE (2005) Oct4 expression in adult human stem cells: evidence in support of the stem cell theory of carcinogenesis. Carcinogenesis 26, 495–502. [DOI] [PubMed] [Google Scholar]
- 48. Wang R, Li J, Yashpal N (2004) Phenotypic analysis of c‐Kit expression in epithelial monolayers derived from postnatal rat pancreatic islets. J. Endocrinol. 182, 113–122. [DOI] [PubMed] [Google Scholar]
- 49. Beltrami AP, Cesselli D, Bergamin N, Marcon P, Rigo S, Puppato E et al. (2007) Multipotent cells can be generated in vitro from several adult human organs (heart, liver and bone marrow). Blood 110, 3438–3446. [DOI] [PubMed] [Google Scholar]
- 50. Yeo S, Jeong S, Kim J, Han JS, Han YM, Kang YK (2007) Characterization of DNA methylation change in stem cell marker genes during differentiation of human embryonic stem cells. Biochem. Biophys. Res. Commun. 359, 536–542. [DOI] [PubMed] [Google Scholar]
- 51. Tay Y, Zhang J, Thomson AM, Lim B, Rigoutsos I (2008) MicroRNAs to Nanog, Oct4 and Sox2 coding regions modulate embryonic stem cell differentiation. Nature 455, 1124–1128. [DOI] [PubMed] [Google Scholar]


