Abstract
Abstract. Since stem cells are present throughout the lifetime of an organism, it is thought that they may accumulate mutations, eventually leading to cancer. In the breast, tumours are predominantly oestrogen and progesterone receptor‐positive (ERα/PR+). We therefore studied the biology of ERα/PR‐positive cells and their relationship to stem cells in normal human mammary epithelium. We demonstrated that ERα/PR‐positive cells co‐express the putative stem cell markers p21CIP1/WAF1, cytokeratin (CK) 19 and Musashi‐1 when examined using dual label immunofluorescence on tissue sections. Next, we isolated a Hoechst dye‐effluxing ‘side population’ (SP) from the epithelium using flow cytometry and demonstrated them to be undifferentiated cells by lack of expression of myoepithelial and luminal cell‐specific antigens such as CALLA and MUC1. Epithelial SP cells were shown to be enriched for the putative stem cell markers p21CIP1/WAF1, Musashi‐1 and ERα/PR‐positive cells. Lastly, SP cells, compared to non‐SP, were highly enriched for the capacity to produce colonies containing multiple lineages in 3D basement membrane (Matrigel) culture. We conclude that breast stem cells include two populations: a primitive ERα/PR‐negative stem cell necessary for development and a shorter term ERα/PR‐positive stem cell necessary for adult tissue homeostasis during menstrual cycling. We speculate these two basic stem cell types may therefore be the cells of origin for ERα‐positive and ‐negative breast tumours.
INTRODUCTION
Tissue‐specific stem cells are defined by their ability to self‐renew and to produce the differentiated, functional cells within an organ. Differentiated cells are generally short‐lived; in skin and blood for example, they are produced from a small pool of long‐lived stem cells that last throughout life. (Dexter & Spooncer 1987; Jones 1997; Watt 1998; Orkin 2000). Thus, stem cells are necessary for tissue development, replacement and repair (Fuchs & Segre 2000). However, stem cell longevity makes them susceptible to accumulating genetic damage and they represent likely targets for carcinogenic transformation. The development, differentiation and function of the mammary gland would not be possible without tissue‐specific stem cells. Full development of mammary epithelium occurs only during pregnancy and lactation to be followed at weaning by involution. The cycle of pregnancy‐associated proliferation, differentiation, apoptosis and remodelling can occur many times during the reproductive lifespan of mammals and may be explained by the presence of a long‐lived population of stem cells that have a near infinite propensity to produce functional cells. One implication of the ‘multi‐hit theory’ of carcinogenesis is that cancer is a stem cell disease, suggesting that successful breast cancer prevention strategies must be targeted to mammary epithelial stem cells.
The resting adult human breast consists of a branching ductal system and terminal ductal lobulo‐alveolar units (TDLUs) or lobules that are the functional glands of the pre‐menopausal breast. Each lobule is lined by a layer of luminal epithelial cells surrounded by a basal layer of myoepithelial cells. TDLUs have been reported to be the site from which most breast tumours originate (Wellings et al. 1975). Furthermore, most breast tumours have the phenotype of luminal epithelial cells (Sorlie et al. 2001). The presence of stem cells in the mammary gland and susceptibility to carcinogens appear related. The greatest concentration of stem cells is in the terminal end bud and alveolar bud structures during pubertal development in rodents and it is during this period that the gland is most sensitive to carcinogens (Russo & Russo 1978a, 1978b). Similar structures exist in the breasts of pre‐pubertal and adolescent women, and it is this age group that suffered the highest rates of breast cancer after irradiation due to the atomic detonations in Japan in 1945 (Dawson 1934; McGregor et al. 1977).
Although breast cancer might be considered a stem cell disease, it is not clear whether the tumours seen clinically retain the characteristics of stem cells. Certainly, like putative stem cells, tumours express mainly markers of luminal cells, not myoepithelial cells (Taylor‐Papadimitriou et al. 1989; Li et al. 1998; 1999, 2000; Sorlie et al. 2001; Korsching et al. 2002). On the other hand, it is feasible that the immortalizing or transforming step may occur during transit amplification or differentiation of stem cell progeny, which may explain why many breast tumours acquire features of differentiation. Indeed, the similarities between cancer cells and normal stem cells, such as increased proliferation potential and self‐renewal ability, were noticed 150 years ago and provide a compelling potential reason why cancer chemotherapy may induce remission, yet rarely cures (Behbod & Rosen 2005). The therapies may affect descendent cells that are irrelevant for the persistence and propagation of the disease, leaving the rarer but more potent cancer stem cell unperturbed. Recently, the prospective isolation of breast tumour stem cells has been reported (Al‐Hajj et al. 2003), followed closely by stem cell identification in other solid tumours such as brain (Singh et al. 2004). This increases the importance of investigating breast stem cell characteristics so that cancer stem cells can be targeted for therapy, preferably without inducing unwanted toxicities in normal stem cells.
Evidence for a breast epithelial stem cell
There is good evidence to suggest that the luminal and the myoepithelial cell types of the normal breast arise from a shared, pluripotent stem cell. Many years ago, it was demonstrated that small fragments of the rodent duct or TEBs transplanted in cleared mammary fat pads of syngeneic hosts could develop an entire and functional mammary tree (DeOme et al. 1959; Hoshino & Gardner 1967; Daniel et al. 1968; Ormerod & Rudland 1986). Serial transplantation, allowing full differentiation each time, revealed that the capacity for regeneration was not infinite, being lost by the seventh transplant generation (Daniel & Young 1971; Young et al. 1971). Surprisingly, the results indicated that the regenerative ability of mammary tissue from old mice was similar to that taken from young mice, and neither reproductive history nor reproductive state affected regenerative capacity. The authors concluded from their experiments that the eventual growth senescence of serially transplanted mammary epithelium was essentially a function of the number of stem cell divisions that had taken place (Daniel & Young 1971). More recently, using viral integration to mark and follow individual clones, it has been reported that a fully differentiated mammary gland can be derived from a single cell clone (Kordon & Smith 1998).
Data demonstrating that the human breast is generated from stem cells have been provided by studies of the pattern of X‐chromosome inactivation throughout the ductal and lobular epithelium. These showed that contiguous patches of epithelium with inactivation of the same X‐chromosome were present, suggesting that the cells within the patch had been derived from the same stem cell (Tsai et al. 1996; Diallo et al. 2001). Further evidence for the existence of human breast stem cells comes from studies showing that the same genetic lesion can be detected throughout an individual lobule or duct within histologically normal mammary epithelium (Deng et al. 1996; Lakhani et al. 1996). Second, separated myoepithelial and luminal cells from the same breast region have similar patterns of loss of heterozygosity suggesting a common progenitor (Lakhani et al. 1999).
Steroid receptor expression patterns
Ovarian steroids play a critical role in mammary gland development and tumourigenesis acting through specific nuclear receptors expressed in target cells. Cells containing receptors for oestrogen and progesterone (ERα and PR, respectively) are located in the luminal epithelium of the ductal and lobular structures (Petersen et al. 1987), and it has been estimated that receptor‐positive cells account for 10–20% of the epithelial cell population. ERα and PR are co‐expressed in luminal epithelial cells but dividing cells are ERα/PR‐negative and often adjacent to ERα/PR‐positive cells (Clarke et al. 1997; Brisken et al. 1998; Russo et al. 1999; Ellis & Capuco 2002). This separation appears to be disrupted early in breast tumourigenesis because actively dividing steroid receptor‐positive tumour cells can be found in premalignant lesions such as atypical ductal hyperplasia (Shoker et al. 1999). Since breast cancers are mainly ERα/PR‐positive, the aim of our studies has been to examine the relationship of ERα/PR‐positive cells to stem cells in normal human breast epithelium.
Intermediate human breast cells express steroid receptors
There is evidence from both rodents and humans that a population of division‐competent mammary epithelial cells of a distinctive morphology can be found in a position intermediate between the basal and luminal cells (Smith et al. 1984; Ferguson 1985; Smith & Medina 1988; Chepko & Smith 1997). These intermediate cells are distinguished by their pale staining cytoplasm under both light and electron microscopy (Chepko & Smith 1997). They have few cellular organelles and pale nuclei and are found as small light cells (SLC) and as undifferentiated large light cells (ULLC). Their infrequent occurrence alone or in pairs and their undifferentiated character has made intermediate cells the focus of suggestions that they may be the mammary stem cell. There is some existing and some emerging evidence supporting this suggestion. First, when mammary epithelial cells from nulliparous mice are placed in culture, it is the pale staining cells that divide first irrespective of whether or not hormones are present in the medium (Smith & Medina 1988). In the presence of the lactogenic hormones insulin, hydrocortisone and prolactin, groups of pale cells gradually disappear and darker cells producing milk proteins appear. In the absence of lactogenic hormones, pale cells remain and no milk proteins are produced (Smith & Medina 1988; Chepko & Smith 1997). More recently, it has been shown that pale staining cells are depleted in growth senescent serial mammary epithelial transplants and their disappearance coincides with growth cessation (Smith et al. 2002). Thus, in rodents the results of in vitro and in vivo experiments suggest that the pale staining or light cells situated between the luminal and the myoepithelial cell layers are the most likely candidates for a stem cell population.
These data seemingly contradict previous results in both human and rodent studies where a stem cell type that is multipotent in culture was demonstrated to be part of the luminal cell population (1998, 2001; Pechoux et al. 1999; Smalley et al. 1999). However, recent work on human breast cells used these contradictory observations to predict that luminal cells that did not contact the lumen would not express the apical membrane‐specific sialomucin MUC1, but would express the luminal epithelial‐specific antigen (ESA). Accordingly, an ESA+/MUC‐1‐population that can give rise to both luminal and myoepithelial cell types in culture has been isolated (Gudjonsson et al. 2002b). These cells were also shown to express cytokeratin (CK) 19 (Gudjonsson et al. 2002b). Therefore, we analysed the CK19 expression and its relationship to proliferating cells and those expressing steroid receptors. In many lobules CK19 expression was homogeneous in that all luminal cells were stained. In others, CK19 expression was scattered throughout the lobule and labelled the cytoplasm of infrequent cells that rarely contained the proliferation‐associated nuclear antigen Ki67. However, these scattered CK19‐positive cells were frequently steroid receptor positive. Second, when the position of ERα/PR‐positive cells in relation to the luminal and myoepithelial cell layers was assessed, three‐quarters of these ERα/PR‐positive cells were in an intermediate position (Clarke et al. 2005).
Mammary epithelial side population (SP) analysis
In order to characterize putative epithelial stem cells further, we digested histologically normal breast tissue obtained from premenopausal women with collagenase and trypsin to obtain a single cell suspension and stained them with Hoechst 33342. Following flow cytometric analysis using a fluorescently labelled epithelial specific antibody (BER‐EP4), an epithelial ‘side population’ (SP) was obtained, averaging 5% of cells that were able to efflux the fluorescent dye (Clarke et al. 2005). Haematopoietic cells that efflux Hoechst 33342 can reconstitute the bone marrow of lethally irradiated mice, suggesting that they are stem cells (Goodell et al. 1997; Jackson et al. 1999; McKinney‐Freeman et al. 2002). The method has also been used to isolate an SP from mouse mammary glands. Mouse mammary SP cells are enriched for expression of three putative stem cell markers; Sca‐1, α6‐integrin and telomerase (Welm et al. 2002; Alvi et al. 2003; Liu et al. 2004). Mouse mammary SP cells were estimated to be 2–3% of epithelial cells in two studies (Liu et al. 2004; Welm et al. 2002) and 0.5% of total cells in the other (Alvi et al. 2003). Alvi et al. have further reported that nearly half of the SP cells were steroid receptor‐positive (Alvi et al. 2003).
A similar SP to that observed in the mouse mammary gland has also been identified by several groups working on normal human breast tissue obtained from reduction mammoplasty and other non‐cancer breast surgery (Alvi et al. 2003; Dontu et al. 2003; Clayton et al. 2004; Clarke et al. 2005). In the three groups who have performed human breast tissue SP analyses, the proportion of breast SP cells varied from ∼0.2% (Alvi et al. 2003; Clayton et al. 2004) to ∼1% (Dontu et al. 2003) to ∼5% (Clarke et al. 2005).
The reason for this variation is partly that the SP cells are not a discrete population but form a continuum with the rest of the cell population, although methodological differences certainly account for some of it. For example, in our study where the highest percentage of SP cells was reported (Clarke et al. 2005), only epithelial cells were included in the flow cytometric analysis by using a fluorescence‐labelled epithelial‐specific antibody (BER‐EP4). One caveat to this method may be that not all epithelial cells were counted since those with low levels of the marker may have been discounted. In each of the other studies (Alvi et al. 2003; Clayton et al. 2004; Clarke et al. 2005), epithelial cells were not separated from other breast cell types using antibody recognition. However, they argue that because only epithelial cell colonies grew out in culture from the isolated SP cells, they must be epithelial. This finding does not exclude the likelihood that non‐epithelial cells were present in the non‐SP. Supporting this, in the study of mouse mammary cells, only 5% of the SP expressed CD45, a haematopoietic cell marker, but 40% of the non‐SP cells were CD45‐positive (Alvi et al. 2003). The actual percentage of SP in the epithelium may therefore be slightly higher than measurements given by Alvi et al. and Clayton et al. (Alvi et al. 2003; Clayton et al. 2004).
Cell culture studies using human breast SP cells
Although different proportions of isolated human breast SP cells were reported in the above studies, their stem cell nature has been analysed and compared to the non‐SP cells using various in vitro cell culture methods. When using human tissue, it has obviously been difficult to test their ‘stem‐ness’ by using transplantation into cleared mouse mammary fat pads because human breast cells do not form a mammary tree as xenografts. However, this approach may be feasible in the near future using a novel method for the humanization of the mouse mammary fat pad (Kuperwasser et al. 2004). Growing SP and non‐SP at clonal densities in monolayer culture in vitro either on feeder layers or on collagen produced three types of colonies: those consisting of myoepithelial or luminal epithelial cells alone and mixed colonies of both cell types. However, depending on the substratum, SP cells produced two to seven times more colonies than non‐SP cells. In support of their putative stem cell nature, only the SP cells possessed the ability to produce colonies with both myoepithelial and luminal epithelial cell types (Clayton et al. 2004; Clarke et al. 2005). Another published method for the culture of undifferentiated tissue‐specific stem cells is the growth of colonies from single cells in non‐adherent suspension culture, such as neurospheres grown from brain tissue, which are enriched in neural stem cells (Dontu et al. 2003). Where this has been applied to human breast cells grown as ‘mammospheres’, 27% of the total population of sphere cells were found to be fall into the SP region following Hoechst staining. Conversely, only SP, and not non‐SP cells, from fresh breast cell digests were capable of forming mammospheres in non‐adherent suspension culture (Dontu et al. 2003). Finally, in three‐dimensional (3D) cultures in basement membrane preparations such as Matrigel (Fred Baker Scientific, Runcorn, UK), breast cells can differentiate to form acini (small hollowed out or solid colonies), or large branching structures reminiscent of lobular structures in vivo. In fact, it has been shown that isolated luminal epithelial cells produce acinus‐like, polarized structures with a hollow lumen, and myoepithelial cells produce a solid acinus (Gudjonsson et al. 2002a). In contrast, single cells with stem/progenitor properties, for example, cells derived from mammospheres or other multipotent breast cells, produce branching lobule‐like structures. We demonstrated in our study that human breast epithelial (BER‐EP4+) SP cells produce branching type structures, while non‐SP cells produced only acinus‐like structures (Clarke et al. 2005). Furthermore, each individual branching structure contained separate populations of cells expressing cytokeratins (CK) of either myoepithelial (CK14) or luminal epithelial (CK18) type.
Stem cell marker expression in breast SP cells
Putative stem cell markers and differentiation markers have been analysed in the SP and compared to the non‐SP cells by two research groups (Clayton et al. 2004; Clarke et al. 2005). In these studies, quantitative reverse transcriptase polymerase chain reaction (QRT‐PCR) was used to assess gene expression, and antibody staining was used to analyse protein synthesis. Using antibodies to the cell surface markers of differentiated myoepithelial and luminal epithelial cells, CALLA and MUC1, respectively, it was demonstrated by both groups that ∼70% of epithelial SP cells expressed neither protein, whereas most non‐SP cells expressed one or the other of these differentiated cell markers (Clayton et al. 2004; Clarke et al. 2005). This result strongly suggests that SP cells include an undifferentiated population of cells. In agreement with this, QRT‐PCR analysis of SP cells by the Clayton group also demonstrated that most SP, but not non‐SP, cells lacked these differentiation markers. Because the breast is a steroid hormone‐responsive tissue, both groups analysed oestrogen receptor (ER) expression. Clarke et al. found a 6‐fold increased ER‐α expression in SP compared to non‐SP cells using both QRT‐PCR and antibody staining, whereas Clayton et al. (2004) found no SP cells expressing either the ER‐α or the ER‐β gene by QRT‐PCR (Clarke). Again, the issue of measuring the proportion of human breast SP cells becomes relevant as these studies had a 25‐fold difference in their analysis (∼5% vs. ∼0.2%), presumably because of the reasons outlined above and in the technical issues section below. This difference may help explain why these gene expression results are directly at odds with each other. On the other hand, Alvi et al. (Alvi et al. 2003) found that up to half of their mouse mammary SP cells expressed ER‐α protein even though their SP fraction was similar in percentage to that of Clayton et al. (Clayton et al. 2004).
Finally, the expression of putative stem cell markers has been demonstrated to be increased in SP compared to non‐SP cells. For example, p21CIP1/WAF1 and Musashi‐1 were reported to be 2‐fold and 6‐fold overexpressed as measured by QRT‐PCR (Clarke et al. 2005). Interestingly, these proteins were co‐expressed with ER‐α in breast epithelial cells examined by dual label immunofluorescence, suggesting that SP cells may express all three proteins (Clarke et al. 2005). The proliferation marker Ki67 was absent in SP cells by QRT‐PCR (Clayton et al. 2004), which would fit with the established fact that cells expressing ER‐α do not proliferate in breast epithelium in vivo (Clarke et al. 1997) and the long‐recognized quiescence of tissue‐specific stem cells.
DNA label‐retaining cells express ER‐α, PR, p21WAF1/CIP1 and Musashi‐1
Two studies of the mouse mammary gland have used DNA label retention as a marker for the stem cell population. The technique has been used previously to label putative skin and intestinal stem cells (Potten & Morris 1988; Potten & Loeffler 1990). It relies on the dilution of DNA label when a cell divides such that it is undetectable after a small number of divisions. Since stem cells are thought to be quiescent compared to their progeny that undergo transit amplification and form the majority of proliferating cells within a tissue, they would be expected to retain label over long periods. In addition, stem cells may retain label through the selective segregation of old and new DNA strands at division as has been shown in the intestinal epithelium and more recently in breast (Cairns 1975; Potten et al. 2002; Smith 2005).
Two groups reported attempts to define mammary gland stem cells in the mouse using injection of DNA label and a pulse chase experiment to characterize label‐retaining cells (LRCs). The studies differed in the DNA label that was used, the developmental stage at which labelling was conducted and the length for which LRCs were followed. Not surprisingly perhaps, the studies produced different results. In the first study, tritiated thymidine (3H‐dT) label was injected into 10–12 week old mice and the LRCs were followed for up to 3 weeks (Zeps et al. 1996, 1998). At 2 weeks, the number of LRCs was small, comprising less than 0.1–1% of epithelial cells depending on the stage of oestrous at injection (Zeps et al. 1996). Interestingly, of the heavily labelled LRCs, 95% expressed oestrogen receptor‐α (ERα), whereas the remaining 5% of LRCs were ERα‐negative basal cells (Zeps et al. 1998). In the second study, the DNA label was bromodeoxyuridine (BrdU) given continuously over a 2‐week period to mice between the ages of 3 and 5 weeks and the LRCs followed until the mice were 13 weeks old (Welm et al. 2002). The period of labelling in this study covers the beginning of puberty in the mouse, and it seems probable that more stem cell division might occur in early puberty than after puberty (1998, 1996). Two weeks following the labelling period in the second study, less than 30% of LRCs expressed progesterone receptor (PR), and at 9 weeks, less than 2% of LRCs were PR‐positive (Welm et al. 2002). No relationship between the strength of DNA label in LRCs and steroid receptor was reported. Some of the LRCs at 9 weeks following labelling were shown to be undifferentiated in character since they lacked cytokeratins 14 and 18 that are indicative of myoepithelial and luminal epithelial cells, respectively (Welm et al. 2002). The data from these two studies can be interpreted as indicating that there are at least two populations of stem cells: a long lived, mainly quiescent, steroid receptor‐negative stem cell that survives throughout the pubertal period, and a shorter lived, less quiescent, steroid receptor‐positive stem cell that is more active during post‐pubertal oestrous cycles.
We studied LRCs in the human breast using an athymic nude mouse model in which the breast tissue is implanted subcutaneously (Clarke et al. 2005). The fate of lobular epithelial cells dividing in response to oestrogen was tracked by administering the DNA label 1 week after the start of oestrogen treatment and observing the LRCs for 2 weeks in the continued presence of the hormone (2 mg oestradiol pellet). We assessed the frequency of staining of LRCs with antibodies to two putative stem cell markers, the CDKI p21WAF1/CIP1 (Topley et al. 1999; Cheng et al. 2000) and a novel stem cell marker, the RNA‐binding protein Musashi‐1 (Msi1) (Sakakibara et al. 1996).
The CDKI p21CIP1 has been proposed to maintain skin and haemopoietic stem cell quiescence since its deletion in p21CIP1 null mice leads to depletion of stem cells in these tissues (Topley et al. 1999; Cheng et al. 2000). Immunohistochemical detection of p21WAF1/CIP1‐positive cells in normal breast lobules indicated that they were infrequent in lobular epithelium (∼0.5%), but 12‐fold more frequent in LRCs (Clarke et al. 2005).
Msi1 is the human homologue of drosophila Musashi protein (Nakamura et al. 1994; Okabe et al. 2001). It is a 362‐amino acid RNA‐binding protein involved in the Delta/Notch signalling pathway that operates during asymmetric cell division (Imai et al. 2001; Okabe et al. 2001; Okano et al. 2002). Msi1 is strongly expressed in murine neural and intestinal stem cells (Sakakibara et al. 1996; Clarke et al. 2003). It is infrequently observed in lobular epithelium and has a distinctive pattern of staining that is punctate and perinuclear in appearance. Msi1 staining is also infrequent in lobular cells (∼0.5%), but 14‐fold more frequent in LRCs (Clarke et al. 2005). Msi1 was expressed at a similar frequency to p21WAF1/CIP1 in lobular epithelial cells and each included cells both intermediate and luminal in position although no cells expressing both these markers were observed, suggesting that they mark separate stem cell populations.
Relationship of putative stem cell markers to steroid receptor expression
The majority of steroid receptor‐positive cells lies in an intermediate position in lobular epithelium and the majority of epithelial SP cells is likely to be intermediate since they express neither the basal marker CALLA nor the apical membrane marker MUC1. Steroid receptor‐positive cells express CK19, a marker previously associated with a stem cell population (Gudjonsson et al. 2002b). We demonstrated in our studies that p21WAF1/CIP1 and Msi1 expression was tightly associated with ERα/PR‐positive cells in immunofluorescent colocalization studies (Clarke et al. 2005). Since Msi1 has been shown to be a positive regulator of Notch signalling through its interaction with Numb mRNA and repression of translation, it has been proposed that Msi1 regulates asymmetric cell division at the stem cell/transit cell boundary through Delta/Notch signalling (Imai et al. 2001). Delta/Notch signalling is an evolutionarily conserved pathway that regulates the stem cell/transit cell boundary in both invertebrate and mammalian tissues (Lowell et al. 2000; Imai et al. 2001; Okabe et al. 2001; Kopan 2002). At this boundary, an asymmetric stem/progenitor cell division specifies one daughter cell to replace the stem/progenitor cell and the other to enter the transit‐amplifying population that multiplies to produce differentiated lineages. This suggests that in cells in which steroid receptors and Msi1 are co‐expressed, numb would be down‐regulated leading to cleavage of the Notch cytoplasmic domain and its translocation to the nucleus where it is known to positively regulate CSL transcription factors (Kopan 2002). We therefore analysed the location of the Notch1 cytoplasmic domain that has been reported to be associated with Msi1 expression in neural stem cells (Kanemura et al. 2001).
We found Notch1 expression was confined mainly to the membrane of epithelial cells where it is inactive. However, in Msi‐positive cells membrane staining was absent suggesting that Notch1 had been cleaved from the membrane and translocated to the nucleus (Clarke et al. 2005). This explanation is in agreement with studies on murine neural cells where Notch1 translocates to the nucleus only in Msi1‐positive stem cells (Imai et al. 2001). These patterns of Msi1 and Notch1 expression suggest that when a breast stem cell divides one daughter cell expresses Msi1 and replaces the stem cell while in the other Msi1‐negative daughter cell, Notch1 remains inactive and the cell undergoes transit amplification before differentiating.
CONCLUSIONS
We have employed several complementary approaches to investigate human breast epithelial stem cells and their relationship to both the proliferative population and steroid receptor expressing cells. Our results are consistent with the hypothesis that normal lobular human breast epithelium is hierarchical in organization, with a small number of scattered quiescent stem cells (detected by p21WAF1/CIP1 and Msi1 staining). These cells may overlap with the SP of potential stem cells which we found to be mainly ‘intermediate’ in character since they lack markers of either the basal myoepithelial cells (detected by CALLA staining) or the luminal cells that have an apical membrane (detected by MUC1 staining). ERα/PR‐positive cells are also intermediate in position, and express CK19, p21WAF1/CIP1 and Msi1. Overall, these data suggest that the pool of potential stem cells includes ERα/PR‐positive cells.
In the normal breast, the production of transit‐amplifying cells and differentiated cell lineages from stem cells is likely to occur through asymmetric cell division where one daughter cell remains a stem cell and the other undergoes transit amplification before differentiating. In both invertebrate and mammalian tissues, asymmetric cell division is regulated by a conserved pathway involving Delta/Notch signalling between daughter cells at the stem cell/transit cell boundary (Lowell et al. 2000; Imai et al. 2001; Okabe et al. 2001; Kopan 2002). Our data suggest Delta/Notch communication between Msi1/steroid receptor‐positive cells and adjacent proliferating cells, perhaps following asymmetric division of a stem cell.
Overall, our data suggest a model where a hierarchy of stem cells produce and regulate the transit‐amplifying population that are destined to differentiate following a small number of cell divisions (Fig. 1). Our model predicts that during breast development, the more primitive stem cells are necessary for the clonal derivation of large areas of epithelium similar to the patches seen in X‐chromosome inactivation studies (Tsai et al. 1996; Diallo et al. 2001). The scattered ‘intermediate’ CK19/Msi1/p21WAF1/CIP1/ERα/PR‐positive stem cells may therefore be necessary for generating differentiated cells within a smaller patch of lobular epithelium in response to the release of hormones during menstrual cycles. A model where there is a hierarchical organization of generally quiescent epithelial stem cells surrounded by proliferating cells and differentiated progeny arranged in patches is similar to that seen in the basal epidermis of the skin (Potten & Morris 1988; Mackenzie 1997; Kolodka et al. 1998; Jensen et al. 1999). We speculate that the stem cells that persist in the breast epithelium during prolonged exposure to menstrual cycles uninterrupted by pregnancy would accumulate genetic changes leading to malignant transformation and this may explain the prevalence of CK19 and steroid receptor‐positive breast tumours.
Figure 1.
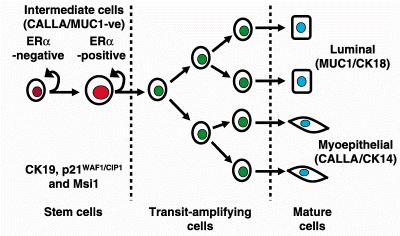
A model of the cellular hierarchies that may exist in the epithelium of the human breast lobule. Cells in an intermediate position include Hoechst dye‐effluxing SP cells and some of these express steroid receptors, p21CIP1, Msi1 and CK19. Msi1 and Delta/Notch signalling may regulate self‐renewal of the stem cell and production of the transit‐amplifying population through a process of asymmetric cell division. After a small number of cell divisions, transit‐amplifying cells exit from the cell cycle and differentiate into myo‐ or luminal epithelial cell lineages characterized by the markers CALLA and CK14 (myoepithelial) or MUC1 and CK18 (luminal).
In summary, we have accumulated evidence to support a model where some steroid receptor‐positive cells act as a stem cell type in the human mammary gland. Clonogenic assays and transplantation studies to confirm their function as long‐lived stem cells remain more difficult than in the rodent. Application of SP isolation in combination with the markers that we have highlighted should provide the tools for future study of the stem cell type that resembles the phenotype of the majority of human breast tumours.
ACKNOWLEDGEMENTS
I am very grateful to colleagues for many useful discussions of this work, and apologise to any researchers whose relevant papers could not be cited due to lack of space. R.B.C. is funded by Cancer Research UK.
REFERENCES
- Al‐Hajj M, Wicha MS, Benito‐Hernandez A, Morrison SJ, Clarke MF (2003) From the cover: prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. U S A 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvi AJ, Clayton H, Joshi C, Enver T, Ashworth A, Vivanco MM, Dale TC, Smalley MJ (2003) Functional and molecular characterisation of mammary side population cells. Breast Cancer Res. 5, R1–R8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behbod F, Rosen JM (2005) Will cancer stem cells provide new therapeutic targets? Carcinogenesis 26, 703–711. [DOI] [PubMed] [Google Scholar]
- Brisken C, Park S, Vass T, Lydon JP, O'Malley BW, Weinberg RA (1998) A paracrine role for the epithelial progesterone receptor in mammary gland development. Proc. Natl Acad. Sci. U S A 95, 5076–5081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns J (1975) Mutation selection and the natural history of cancer. Nature 255, 197–200. [DOI] [PubMed] [Google Scholar]
- Cheng T, Rodrigues N, Shen H, Yang Y, Dombkowski D, Sykes M, Scadden DT (2000) Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808. [DOI] [PubMed] [Google Scholar]
- Chepko G, Smith GH (1997) Three division‐competent, structurally‐distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29, 239–253. [DOI] [PubMed] [Google Scholar]
- Clarke RB, Anderson E, Howell A, Potten CS (2003) Regulation of human breast epithelial stem cells. Cell Proliferation 36, 45–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke RB, Howell A, Potten CS, Anderson E (1997) Dissociation between steroid receptor expression and cell proliferation in the human breast. Cancer Res. 57, 4987–4991. [PubMed] [Google Scholar]
- Clarke RB, Spence K, Anderson E, Howell A, Okano H, Potten CS (2005) A putative human breast stem cell population is enriched for steroid receptor‐positive cells. Dev. Biol. 277, 443–456. [DOI] [PubMed] [Google Scholar]
- Clayton H, Titley I, Vivanco M (2004) Growth and differentiation of progenitor/stem cells derived from the human mammary gland. Exp. Cell Res. 297, 444–460. [DOI] [PubMed] [Google Scholar]
- Daniel CW, De Ome KB, Young JT, Blair PB, Faulkin LJ Jr (1968) The in vivo life span of normal and preneoplastic mouse mammary glands: a serial transplantation study. Proc. Natl Acad. Sci. U S A 61, 53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel CW, Young LJ (1971) Influence of cell division on an aging process. Life span of mouse mammary epithelium during serial propagation in vivo . Exp. Cell Res. 65, 27–32. [DOI] [PubMed] [Google Scholar]
- Dawson EK (1934) A histological study of the normal mamma in relation to tumour growth. Edinburgh Med. J. 41, 653–682. [PMC free article] [PubMed] [Google Scholar]
- Deng G, Lu Y, Zlotnikov G, Thor AD, Smith HS (1996) Loss of heterozygosity in normal tissue adjacent to breast carcinomas. Science 274, 2057–2059. [DOI] [PubMed] [Google Scholar]
- DeOme KB, Faulkin LJ Jr, Bern HA, Blair PB (1959) Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland‐free mammary fat pads of female C3H mice. Cancer Res. 19, 515–520. [PubMed] [Google Scholar]
- Dexter TM, Spooncer E (1987) Growth and differentiation in the hemopoietic system. Annu. Rev. Cell Biol. 3, 423–441. [DOI] [PubMed] [Google Scholar]
- Diallo R., Schaefer KL, Poremba C, Shivazi N, Willmann V, Buerger H, Dockhorn‐Dworniczak B, Boecker W (2001) Monoclonality in normal epithelium and in hyperplastic and neoplastic lesions of the breast. J. Pathol. 193, 27–32. [DOI] [PubMed] [Google Scholar]
- Dontu G, Abdallah WM, Foley JM, Jackson KW, Clarke MF, Kawamura MJ, Wicha MS (2003) In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis S, Capuco AV (2002) Cell proliferation in bovine mammary epithelium: identification of the primary proliferative cell population. Tissue Cell 34, 155–163. [DOI] [PubMed] [Google Scholar]
- Ferguson DJ (1985) Ultrastructural characterisation of the proliferative (stem?) cells within the parenchyma of the normal ‘resting’ breast. Virchows Arch. A Pathol. Anat. Histopathol. 407, 379–385. [DOI] [PubMed] [Google Scholar]
- Fuchs E, Segre JA (2000) Stem cells: a new lease on life. Cell 100, 143–155. [DOI] [PubMed] [Google Scholar]
- Goodell MA, Rosenzweig M, Kim H, Marks DF, Demaria M, Paradis G, Grupp SA, Sieff CA, Mulligan RC, Johnson RP (1997) Dye efflux studies suggest that hematopoietic stem cells expressing low or undetectable levels of CD34 antigen exist in multiple species. Nat. Med. 3, 1337–1345. [DOI] [PubMed] [Google Scholar]
- Gudjonsson T, Ronnov‐Jessen L, Villadsen R., Rank F, Bissell MJ, Petersen OW (2002a) Normal and tumor‐derived myoepithelial cells differ in their ability to interact with luminal breast epithelial cells for polarity and basement membrane deposition. J. Cell Sci. 115, 39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudjonsson T, Villadsen R., Nielsen HL, Ronnov‐Jessen L, Bissell MJ, Petersen OW (2002b) Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino K, Gardner WU (1967) Transplantability and life span of mammary gland during serial transplantation in mice. Nature 213, 193–194. [DOI] [PubMed] [Google Scholar]
- Imai T, Tokunaga A, Yoshida T, Hashimoto M, Mikoshiba K, Weinmaster G, Nakafuku M, Okano H (2001) The neural RNA‐binding protein Musashi1 translationally regulates mammalian numb gene expression by interacting with its mRNA. Mol. Cell Biol. 21, 3888–3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson KA, Mi T, Goodell MA (1999) Hematopoietic potential of stem cells isolated from murine skeletal muscle. Proc. Natl Acad. Sci. U S A 96, 14482–14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen UB, Lowell S, Watt FM (1999) The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole‐mount labelling and lineage analysis. Development 126, 2409–2418. [DOI] [PubMed] [Google Scholar]
- Jones PH (1997) Epithelial stem cells. Bioessays 19, 683–690. [DOI] [PubMed] [Google Scholar]
- Kanemura Y, Mori K, Sakakibara S, Fujikawa H, Hayashi H, Nakano A, Matsumoto T, Tamura K, Imai T, Ohnishi T, Fushiki S, Nakamura Y, Yamasaki M, Okano H, Arita N (2001) Musashi1, an evolutionarily conserved neural RNA‐binding protein, is a versatile marker of human glioma cells in determining their cellular origin, malignancy, and proliferative activity. Differentiation 68, 141–152. [DOI] [PubMed] [Google Scholar]
- Kolodka TM, Garlick JA, Taichman LB (1998) Evidence for keratinocyte stem cells in vitro: long term engraftment and persistence of transgene expression from retrovirus‐transduced keratinocytes. Proc. Natl Acad. Sci. U S A 95, 4356–4361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopan R (2002) Notch: a membrane‐bound transcription factor. J. Cell Sci. 115, 1095–1097. [DOI] [PubMed] [Google Scholar]
- Kordon EC, Smith GH (1998) An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921–1930. [DOI] [PubMed] [Google Scholar]
- Korsching E, Packeisen J, Agelopoulos K, Eisenacher M, Voss R, Isola J, van Diest PJ, Brandt B, Boecker W, Buerger H (2002) Cytogenetic alterations and cytokeratin expression patterns in breast cancer: integrating a new model of breast differentiation into cytogenetic pathways of breast carcinogenesis. Lab. Invest. 82, 1525–1533. [DOI] [PubMed] [Google Scholar]
- Kuperwasser C, Chavarria T, Wu M, Magrane G, Gray JW, Carey L, Richardson A, Weinberg RA (2004) Reconstruction of functionally normal and malignant human breast tissues in mice. Proc. Natl Acad. Sci. U S A 101, 4966–4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakhani SR, Chaggar R., Davies S, Jones C, Collins N, Odel C, Stratton MR, O'Hare MJ (1999) Genetic alterations in ‘normal’ luminal and myoepithelial cells of the breast. J. Pathol. 189, 496–503. [DOI] [PubMed] [Google Scholar]
- Lakhani SR, Slack DN, Hamoudi RA, Collins N, Stratton MR, Sloane JP (1996) Detection of allelic imbalance indicates that a proportion of mammary hyperplasia of usual type are clonal, neoplastic proliferations. Lab. Invest. 74, 129–135. [PubMed] [Google Scholar]
- Li P, Barraclough R., Fernig DG, Smith JA, Rudland PS (1998) Stem cells in breast epithelia. Int. J. Exp. Pathol. 79, 193–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu BY, McDermott SP, Khwaja SS, Alexander CM (2004) The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl Acad. Sci. U S A 101, 4158–4163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowell S, Jones P, Le Roux I, Dunne J, Watt FM (2000) Stimulation of human epidermal differentiation by delta‐notch signalling at the boundaries of stem‐cell clusters. Curr. Biol. 10, 491–500. [DOI] [PubMed] [Google Scholar]
- Mackenzie IC (1997) Retroviral transduction of murine epidermal stem cells demonstrates clonal units of epidermal structure. J. Invest Dermatol. 109, 377–383. [DOI] [PubMed] [Google Scholar]
- McGregor H, Land CE, Choi K, Tokuoka S, Liu PI, Wakabayashi T, Beebe GW (1977) Breast cancer incidence among atomic bomb survivors, Hiroshima and Nagasaki, 1950–69. J. Natl Cancer Inst. 59, 799–811. [DOI] [PubMed] [Google Scholar]
- McKinney‐Freeman SL, Jackson KA, Camargo FD, Ferrari G, Mavilio F, Goodell MA (2002) Muscle‐derived hematopoietic stem cells are hematopoietic in origin. Proc. Natl Acad. Sci. U S A 99, 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M, Okano H, Blendy JA, Montell C (1994) Musashi, a neural RNA‐binding protein required for Drosophila adult external sensory organ development. Neuron 13, 67–81. [DOI] [PubMed] [Google Scholar]
- Okabe M, Imai T, Kurusu M, Hiromi Y, Okano H (2001) Translational repression determines a neuronal potential in Drosophila asymmetric cell division. Nature 411, 94–98. [DOI] [PubMed] [Google Scholar]
- Okano H, Imai T, Okabe M (2002) Musashi: a translational regulator of cell fate. J. Cell Sci. 115, 1355–1359. [DOI] [PubMed] [Google Scholar]
- Orkin SH (2000) Diversification of haematopoietic stem cells to specific lineages. Nat. Rev. Genet. 1, 57–64. [DOI] [PubMed] [Google Scholar]
- Ormerod EJ, Rudland PS (1986) Regeneration of mammary glands in vivo from isolated mammary ducts. J. Embryol. Exp. Morphol. 96, 229–243. [PubMed] [Google Scholar]
- Pechoux C, Gudjonsson T, Ronnov‐Jessen L, Bissell MJ, Petersen OW (1999) Human mammary luminal epithelial cells contain progenitors to myoepithelial cells. Dev. Biol. 206, 88–99. [DOI] [PubMed] [Google Scholar]
- Perou CM, Jeffrey SS, van de Rijn M, Rees CA, Eisen MB, Ross DT, Pergamenschikov A, Williams CF, Zhu SX, Lee JC, Lashkari D, Shalon D, Brown PO, Botstein D (1999) Distinctive gene expression patterns in human mammary epithelial cells and breast cancers. Proc. Natl Acad. Sci. U S A 96, 9212–9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perou CM, Sorlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, Pollack JR, Ross DT, Johnsen H, Akslen LA, Fluge O, Pergamenschikov A, Williams C, Zhu SX, Lonning PE, Borresen‐Dale AL, Brown PO, Botstein D (2000) Molecular portraits of human breast tumours. Nature 406, 747–752. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Hoyer PE, van Deurs B (1987) Frequency and distribution of estrogen receptor‐positive cells in normal, nonlactating human breast tissue. Cancer Res. 47, 5748–5751. [PubMed] [Google Scholar]
- Potten CS, Loeffler M (1990) Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development 110, 1001–1020. [DOI] [PubMed] [Google Scholar]
- Potten CS, Morris RJ (1988) Epithelial stem cells in vivo . J. Cell Sci. Suppl. 10, 45–62. [DOI] [PubMed] [Google Scholar]
- Potten CS, Owen G, Booth D (2002) Intestinal stem cells protect their genome by selective segregation of template DNA strands. J. Cell Sci. 115, 2381–2388. [DOI] [PubMed] [Google Scholar]
- Russo J, Ao X, Grill C, Russo IH (1999) Pattern of distribution of cells positive for estrogen receptor alpha and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res. Treat 53, 217–227. [DOI] [PubMed] [Google Scholar]
- Russo IH, Russo J (1978a) Developmental stage of the rat mammary gland as determinant of its susceptibility to 7,12‐dimethylbenz[a]anthracene. J. Natl Cancer Inst. 61, 1439–1449. [PubMed] [Google Scholar]
- Russo J, Russo IH (1978b) DNA labeling index and structure of the rat mammary gland as determinants of its susceptibility to carcinogenesis. J. Natl Cancer Inst. 61, 1451–1459. [PubMed] [Google Scholar]
- Sakakibara S, Imai T, Hamaguchi K, Okabe M, Aruga J, Nakajima K, Yasutomi D, Nagata T, Kurihara Y, Uesugi S, Miyata T, Ogawa M, Mikoshiba K, Okano H (1996) Mouse‐Musashi‐1, a neural RNA‐binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176, 230–242. [DOI] [PubMed] [Google Scholar]
- Shoker BS, Jarvis C, Clarke RB, Anderson E, Hewlett J, Davies MP, Sibson DR, Sloane JP (1999) Estrogen receptor‐positive proliferating cells in the normal and precancerous breast. Am. J. Pathol. 155, 1811–1815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, Henkelman RM, Cusimano MD, Dirks PB (2004) Identification of human brain tumour initiating cells. Nature 432, 396–401. [DOI] [PubMed] [Google Scholar]
- Smalley MJ, Titley J, Paterson H, Perusinghe N, Clarke C, O'Hare MJ (1999) Differentiation of separated mouse mammary luminal epithelial and myoepithelial cells cultured on EHS matrix analyzed by indirect immunofluorescence of cytoskeletal antigens. J. Histochem. Cytochem. 47, 1513–1524. [DOI] [PubMed] [Google Scholar]
- Smith GH (2005) Label‐retaining epithelial cells in mouse mammary gland divide asymmetrically and retain their template DNA strands. Development 132, 681–687. [DOI] [PubMed] [Google Scholar]
- Smith GH, Medina D (1988) A morphologically distinct candidate for an epithelial stem cell in mouse mammary gland. J. Cell Sci. 90, 173–183. [DOI] [PubMed] [Google Scholar]
- Smith CA, Monaghan P, Neville AM (1984) Basal clear cells of the normal human breast. Virchows Arch. A Pathol. Anat. Histopathol. 402, 319–329. [DOI] [PubMed] [Google Scholar]
- Smith GH, Strickland P, Daniel CW (2002) Putative epithelial stem cell loss corresponds with mammary growth senescence. Cell Tissue Res. 310, 313–320. [DOI] [PubMed] [Google Scholar]
- Sorlie T, Perou CM, Tibshirani R., Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, Thorsen T, Quist H, Matese JC, Brown PO, Botstein D, Eystein Lonning P, Borresen‐Dale AL (2001) Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. U S A 98, 10869–10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Kuusk U, Emerman JT (1998) Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63, 201–213. [DOI] [PubMed] [Google Scholar]
- Stingl J, Eaves CJ, Zandieh I, Emerman JT (2001) Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat 67, 93–109. [DOI] [PubMed] [Google Scholar]
- Taylor‐Papadimitriou J, Stampfer M, Bartek J, Lewis A, Boshell M, Lane EB, Leigh IM (1989) Keratin expression in human mammary epithelial cells cultured from normal and malignant tissue: relation to in vivo phenotypes and influence of medium. J. Cell Sci. 94, 403–413. [DOI] [PubMed] [Google Scholar]
- Topley GI, Okuyama R., Gonzales JG, Con Ti C, Dotto GP (1999) p21 (WAF1/Cip1) functions as a suppressor of malignant skin tumor formation and a determinant of keratinocyte stem‐cell potential. Proc. Natl Acad. Sci. U S A 96, 9089–9094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai YC, Lu Y, Nichols PW, Zlotnikov G, Jones PA, Smith HS (1996) Contiguous patches of normal human mammary epithelium derived from a single stem cell: implications for breast carcinogenesis. Cancer Res. 56, 402–404. [PubMed] [Google Scholar]
- Watt FM (1998) Epidermal stem cells: markers, patterning and the control of stem cell fate. Philos. Trans. R. Soc. Lond. B Biol. Sci. 353, 831–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellings SR, Jensen HM, Marcum RG (1975) An atlas of subgross pathology of the human breast with special reference to possible precancerous lesions. J. Natl Cancer Inst. 55, 231–273. [PubMed] [Google Scholar]
- Welm BE, Tepera SB, Venezia T, Graubert TA, Rosen JM, Goodell MA (2002) Sca‐1(pos) cells in the mouse mammary gland represent an enriched progenitor cell population. Dev. Biol. 245, 42–56. [DOI] [PubMed] [Google Scholar]
- Young LJ, Medina D, Deome KB, Daniel CW (1971) The influence of host and tissue age on life span and growth rate of serially transplanted mouse mammary gland. Exp. Gerontol. 6, 49–56. [DOI] [PubMed] [Google Scholar]
- Zeps N, Bentel JM, Papadimitriou JM, D’Antuono MF, Dawkins HJ (1998) Estrogen receptor‐negative epithelial cells in mouse mammary gland development and growth. Differentiation 62, 221–226. [DOI] [PubMed] [Google Scholar]
- Zeps N, Dawkins HJ, Papadimitriou JM, Redmond SL, Walters MI (1996) Detection of a population of long‐lived cells in mammary epithelium of the mouse. Cell Tissue Res. 286, 525–536. [DOI] [PubMed] [Google Scholar]


