Figure 1.
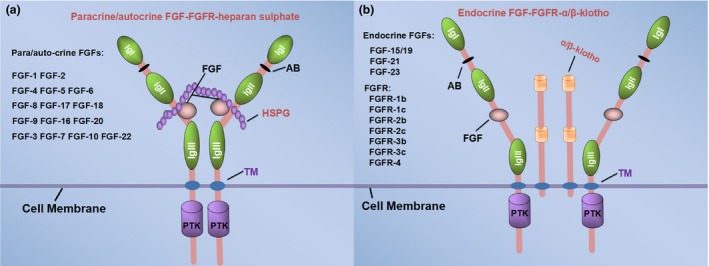
The different binding modes of paracrine and endocrine FGF ligands. The FGFs family are grouped into five paracrine–autocrine‐acting subfamilies (a) and one endocrine‐acting subfamily (b). FGF ligands is required for binding the cofactors to the receptors, and the cofactor of paracrine–autocrine FGF ligands is generally heparan sulphate proteoglycans (HSPGs), while Klotho is the co‐receptor of endocrine FGF ligands. The structure of FGFRs comprises three extracellular immunoglobulin‐like domains (Ig I to III), a transmembrane domain (TM) and an intracellular protein tyrosine kinase domain (PTK).118 The binding sites of FGFs and FGFRs located between IgII and Ig III, and the acid box (AB) composed of eight consecutive acidic residues located between IgI and Ig II. The extracellular domain of FGFRs generates the IIIb and IIIc isoforms through the alternative splicing.119, 120, 121 FGFR‐1, FGFR‐2 and FGFR‐3 all have different isoforms except FGFR‐4 (b). (These FGFRs in (b) are not endocrine FGF specific receptors, which binding with FGFs regardless of paracrine–autocrine or endocrine.) The binding of receptors with ligands leads to dimerization and activation of the tyrosine kinase domain
