High-throughput screening of transposon insertion libraries is a useful strategy for unveiling bacterial genes whose inactivation results in an altered susceptibility to antibiotics. A potential drawback of these studies is they are usually based on just one model antibiotic for each structural family, under the assumption that the results can be extrapolated to all members of said family.
KEYWORDS: intrinsic resistome, Pseudomonas aeruginosa, adjuvants, virulence
ABSTRACT
High-throughput screening of transposon insertion libraries is a useful strategy for unveiling bacterial genes whose inactivation results in an altered susceptibility to antibiotics. A potential drawback of these studies is they are usually based on just one model antibiotic for each structural family, under the assumption that the results can be extrapolated to all members of said family. To determine if this simplification is appropriate, we have analyzed the susceptibility of mutants of Pseudomonas aeruginosa to four aminoglycosides. Our results indicate that each mutation produces different effects on susceptibility to the tested aminoglycosides, with only two mutants showing similar changes in the susceptibility to all studied aminoglycosides. This indicates that the role of a particular gene in the resistome of a given antibiotic should not be generalized to other members of the same structural family. Five aminoglycoside-hypersusceptible mutants inactivating glnD, hflK, PA2798, PA3016, and hpf were chosen for further analysis in order to elucidate if lower aminoglycoside susceptibility correlates with cross-hypersusceptibility to other antibiotics and with impaired virulence. Our results indicate that glnD inactivation leads to increased cross-susceptibility to different antibiotics. The mutant in this gene is strongly impaired in virulence traits such as pyocyanin production, biofilm formation, elastase activity, and swarming motility and the ability to kill Caenorhabditis elegans. Thus, GlnD might be an interesting target for developing antibiotic coadjuvants with antiresistance and antivirulence properties against P. aeruginosa.
INTRODUCTION
Pseudomonas aeruginosa is an opportunistic pathogen, widely distributed in nature (1), which causes a variety of nosocomial infections. It is the main cause of chronic infections in patients with cystic fibrosis (CF) or patients afflicted by chronic obstructive pulmonary disease (2, 3). These infections are usually treated with a set of antibiotics that include β-lactams, polymyxins, and aminoglycosides (4), such as tobramycin or amikacin (5, 6). Acquisition of antibiotic-inactivating enzymes through horizontal gene transfer is fundamental in the development of antibiotic resistance by P. aeruginosa (7). In addition, resistant mutants are frequently selected during antibiotic treatment, particularly in the case of chronic infections (8–10), which hinder the efficacy of antipseudomonal therapy. Identification of the elements that contribute to a reduced susceptibility to antibiotics, as well as those whose inactivation increases resistance, is relevant for understanding the mechanisms involved in P. aeruginosa antibiotic resistance. To this end, high-throughput screening of transposon insertion mutants in search for mutants presenting altered susceptibilities to antimicrobial agents has been shown to be a fruitful strategy (11, 12). Different studies based in the use of transposon-insertion mutants have identified P. aeruginosa genes whose inactivation modifies the susceptibility to antibiotics of this pathogen (13–18). However, these studies are frequently performed using only one antibiotic for each studied group, under the assumption that the results can be extrapolated to other members of the same structural family of antimicrobials (14, 17–19). In the current article, we challenge this hypothesis by analyzing the susceptibility of a large set of P. aeruginosa mutants to four aminoglycosides, namely, tobramycin, amikacin, streptomycin, and kanamycin, by using data obtained from in-house screening and previously published data (16, 18). Our studies may help to elucidate whether the use of one antibiotic provides enough information to conclusively assess the implication of a gene in resistance to a whole family of antimicrobials. It is important to highlight that this type of generalization is applied only in the case of mutations in the elements of the intrinsic resistome and not in the case of the acquisition of antibiotic resistance genes. Indeed, it is known that antibiotic-inactivating enzymes (and, in particular, aminoglycoside-inactivating enzymes) are antibiotic specific. We and others have shown that inactivation of the genes that constitute the intrinsic resistome usually produces pleiotropic effects on the susceptibility to antibiotics from different families (17, 20). Consequently, an additional objective of this study was to identify mutants with cross-hypersusceptibility to antibiotics from different structural families. Since inactivation of these genes increases the susceptibility to different antimicrobials, they could be considered suitable targets for the search of antibiotics' coadjuvants.
Taking into consideration that the ever-increasing burden of resistance erodes the efficacy of conventional antibiotics, copious efforts are being made to develop novel therapeutics that block virulence mechanisms, to be used alone or in combination with classical antibiotics (21, 22). The characterization of targets whose inhibition could simultaneously suppress the expression of virulence factors and antibiotic resistance might fuel a field (23) that remains incipient, in spite of recent studies with exactly this focus (24). In this study, we characterized a set of aminoglycoside-hypersusceptible mutants to identify hypothetical connections between hypersusceptibility and impaired virulence. The genes inactivated in these mutants encode potential targets for the development of P. aeruginosa antivirulence/antiresistance coadjuvants.
RESULTS
The intrinsic resistome of P. aeruginosa to amikacin.
An ordered, comprehensive, nonredundant PA14 transposon insertion library (25) was screened to find genes whose inactivation modifies P. aeruginosa susceptibility to amikacin. From this screening, 118 mutants displayed changes in their susceptibility to amikacin of at least 2-fold, as determined using an agar dilution method (15, 26). These results included bona fide intrinsic resistance genes (genes that contribute to the characteristic phenotype of P. aeruginosa susceptibility to amikacin) and genes whose mutation results in low-level amikacin resistance in this pathogen (see Table S1 in the supplemental material).
The shared intrinsic resistome to aminoglycosides of P. aeruginosa.
Several publications on the intrinsic resistome of bacterial pathogens analyzed only one antibiotic belonging to each structural family in the understanding that the data obtained with one antibiotic might be extrapolated to other members in the family (14, 17–19). To decipher whether or not this was indeed the situation in our case, we included in the analysis genes whose inactivation has been previously reported to modify P. aeruginosa susceptibility to aminoglycosides (16, 18). The final number of selected genes was 243 (Table S1). Strains 1 to 118 are the mutants provided from our screening, whereas strains 119 to 122 came from Krahn’s work (16) and strains 123 to 243 from Schurek’s (18). We chose to use the PAO1 codes of the orthologue counterparts to name the PA14 screened mutants.
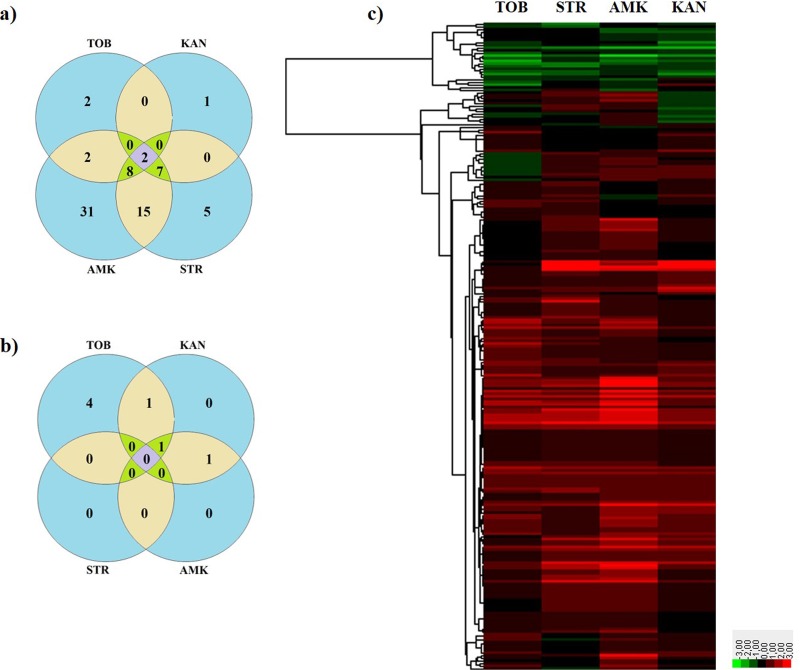
The susceptibility of these 243 P. aeruginosa PA14 insertion mutants to tobramycin, amikacin, streptomycin, and kanamycin was determined using MIC strips. As shown, 80 transposon-tagged insertion mutants showed changes of at least 3-fold compared to the wild-type strain in their susceptibility to at least one aminoglycoside. Among these, only two mutants showed changes in the MICS of all four studied aminoglycosides (the ones inactivating pilC and clpS, the latter being already related to the intrinsic resistome to aminoglycosides of P. aeruginosa [27]), 16 in the MICs of three, 19 in the MICs of two, and 43 in the MIC of one (Fig. 1a and b). Notably, these changes were not equally distributed for all aminoglycosides/mutants, and a variety of susceptibility patterns was observed among the studied mutants (Fig. 1c). These results suggest that a change in susceptibility to one antimicrobial agent associated with the inactivation of a given gene does not necessarily imply a similar change in the phenotype of susceptibility to other antibiotics, even when the latter belong to the same structural family.
FIG 1.
Susceptibility to four aminoglycosides of P. aeruginosa mutants. (a and b) Venn diagrams with the number of mutants with changes in their susceptibility to the four aminoglycosides 3-fold above (a) or below (b) the MIC of the parental strain. (c) Hierarchical clustering of the MICs obtained from the aminoglycoside susceptibility screening of 243 P. aeruginosa PA14 transposon insertion mutants. The values were represented as log2 [MICmutant/MICPA14], using Gene Cluster 3.0 software and Java Treeview for the graphic display. TOB, tobramycin; KAN, kanamycin. STR, streptomycin; AMK, amikacin. Green represents increased susceptibility and red reduced susceptibility.
Cross-susceptibility of P. aeruginosa aminoglycoside-hypersusceptible mutants.
Among the P. aeruginosa PA14 transposon insertion mutants, we selected for further analysis those that exhibited at least a 3-fold increase in susceptibility to at least one aminoglycoside compared to the wild-type strain. The mutants with mutations in genes PA3658, PA4942, PA2798, PA3016, and PA4463 met those requirements. These genes encode GlnD (a protein implicated in N2 metabolism) (28), HflK (an FtsH protease accessory factor) (29), a probable two-component regulator, a hypothetical protein already described to be involved in intrinsic aminoglycoside resistance (30), and a hibernation-promoting factor (Hpf) that is required for rRNA preservation during starvation (31, 32), respectively (Table S1). Two other mutants, one with a mutation in mucD and another with a mutation in amgS, were also hypersusceptible but were excluded from further analysis. The mucD mutant was excluded because it exhibited an increase in susceptibility to tobramycin and a decrease in susceptibility to kanamycin (Table S1). The role of amgS in virulence and resistance to different antibiotics, namely, aminoglycosides, macrolides, quinolones, and β-lactams (30, 33, 34), as well as the nexus between the two-component system it belongs to and the aminoglycoside-promoted expression of the multidrug efflux pump MexXY (35), has already been analyzed in detail and further analysis would thus be redundant. To further confirm the presence of the transposon in these genes, the regions holding it were amplified using specific oligonucleotides (Table S2). To determine if the selected mutants were cross-hypersusceptible to other antibiotics, the MICs of a set of representative antimicrobials were determined. Every mutant, except for the PA3016 mutant, showed higher susceptibility to antibiotics from different structural families (Table 1), implying that the effect of the inactivated genes on antibiotic resistance may not be aminoglycoside specific. All the mutants, except for the PA3016 mutant, presented increased susceptibility to fosfomycin. In addition, all the mutants, except for PA3016 and PA4942, exhibited an increase in susceptibility to tigecycline and tetracycline. The PA3658 mutant displayed the most hypersusceptible phenotype.
TABLE 1.
MICs of antibiotics of different structural families in the selected P. aeruginosa PA14 aminoglycoside-hypersusceptible mutants
| Mutant | MIC (μg/ml)a |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| TGC | TET | CIP | CAZ | IPM | ATM | FOF | ERY | CHL | |
| PA14 | 8.0 | 2.0 | 0.125 | 1.0 | 0.75 | 2.0 | 8.0 | 96.0 | 24.0 |
| PA3016 | 6.0 | 1.5 | 0.094 | 1.5 | 0.75 | 3.0 | 6.0 | 96.0 | 24.0 |
| hpf | 4.0 | 0.38 | 0.094 | 0.5 | 0.75 | 2.0 | 3.0 | 48.0 | 24.0 |
| PA2798 | 3.0 | 0.5 | 0.064 | 1.0 | 0.75 | 2.0 | 1.5 | 48.0 | 16.0 |
| hflK | 6.0 | 1.5 | 0.125 | 0.75 | 0.75 | 1.0 | 4.0 | 96.0 | 24.0 |
| glnD | 0.75 | 0.25 | 0.064 | 0.75 | 0.75 | 1.5 | 4.0 | 64.0 | 24.0 |
TGC, tigecycline; TET, tetracycline; CIP, ciprofloxacin; CAZ, ceftazidime; IPM, imipenem; ATM, aztreonam; FOF, fosfomycin; ERY, erythromycin; CHL, chloramphenicol.
P. aeruginosa aminoglycoside-hypersusceptible mutants are impaired in their virulence potential.
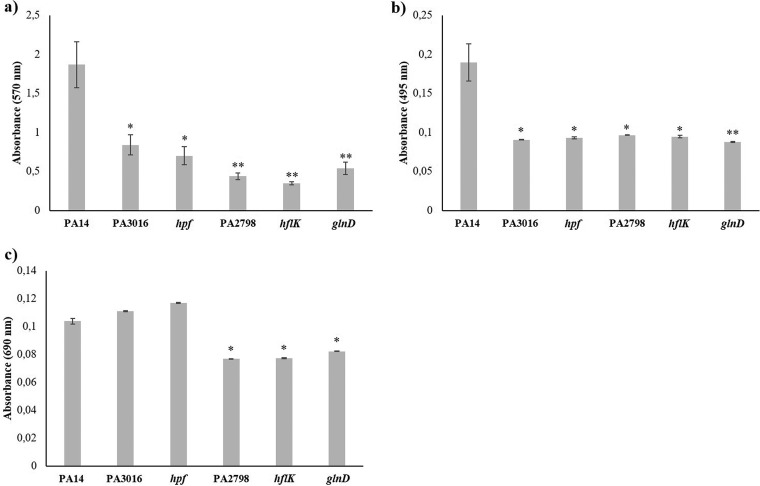
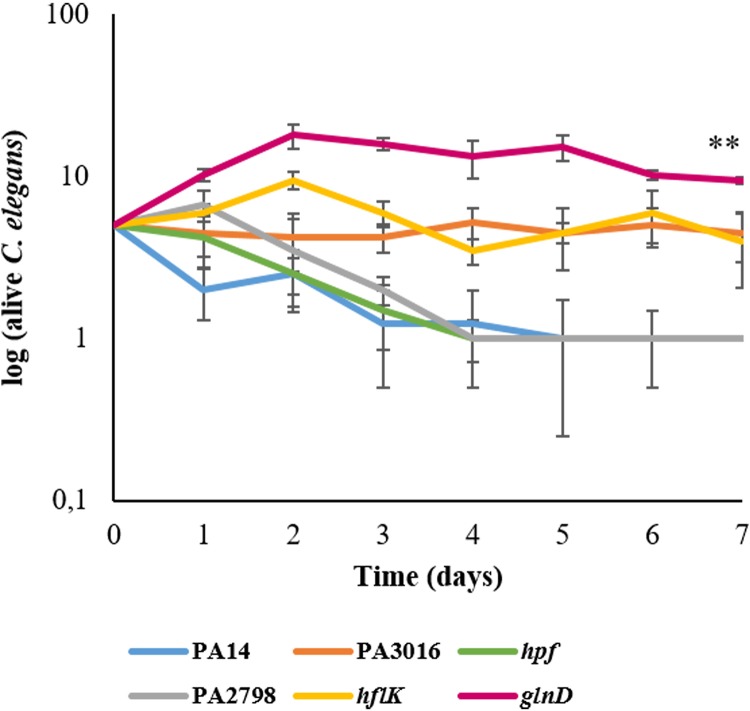
Besides contributing to P. aeruginosa intrinsic antibiotic resistance, the analyzed genes might also impact the production of elements relevant to infection by this bacterial pathogen. To address this possibility, levels of biofilm, elastase, and pyocyanin production, as well as swarming motility phenotypes, were compared between the mutants and the wild-type strain. All the mutants were impaired in biofilm formation, a situation that was especially remarkable in the case of the PA3658, PA2798, and PA4942 mutants (Fig. 2). Additionally, all the mutants exhibited lower elastinolytic activity; however, in this case, the levels of all the mutants were akin (Fig. 2). Concerning pyocyanin synthesis, the level of impairment displayed by the mutants was lower. The PA3016 and PA4463 mutants maintained levels of pyocyanin production similar to those maintained by the parental strain, whereas the mutants with mutations in PA2798, PA3658, and PA4942 showed moderate and yet statistically significant reductions in such production (Fig. 2). Swarming motility was also lessened, with the PA3658 and PA2798 mutants (also largely impaired in biofilm formation and elastinolytic activity) presenting the most anomalous motility patterns (Fig. 3). To determine whether the lower production of virulence factors by the aminoglycoside-hypersusceptible mutants correlates to impaired virulence in an infection model, we performed a Caenorhabditis elegans killing assay. As shown in Fig. 4, P. aeruginosa PA14 and the PA2798 and PA4463 mutants were the most lethal to C. elegans; almost the entire population of worms died within 4 to 5 days. In contrast, the PA3658 mutant appeared to be less lethal, with 9 to 11 nematodes still alive at the end of the experiment. The PA4942 and PA3016 mutants also presented a less lethal phenotype than P. aeruginosa PA14, albeit the results were not as clear as the ones obtained with the PA3658 mutant.
FIG 2.
Quantification of different phenotypes with relevance for the virulence of P. aeruginosa in aminoglycoside-hypersusceptible mutants. The graphs show (a) biofilm formation assay data, (b) elastase activity data, and (c) pyocyanin production of P. aeruginosa PA14 and aminoglycoside-hypersusceptible mutants selected from the screening of the transposon insertion library of this strain. Error bars indicate standard deviations of the results from eight independent experiments in the biofilm formation assay and from three experiments in the other tests. Statistically significant differences with P values of <0.05 in the assayed features with respect to the wild-type strain were evaluated using Student's t test and are highlighted with one asterisk, whereas P values of <0.005 are indicated with two asterisks.
FIG 3.
Swarming assay of P. aeruginosa PA14 and aminoglycoside-hypersusceptible mutants. The figure shows the swarming of a set of selected mutants in comparison with the PA14 wild-type strain. Three replicates of each mutant were assayed, and pictures were taken after 17 h of incubation at 37°C. The diameters displayed represent means of results from the three replicates.
FIG 4.
Virulence of P. aeruginosa aminoglycoside-hypersusceptible mutants in a C. elegans model system. Data present the growth kinetics of C. elegans in the presence of either the PA14 wild-type strain or the P. aeruginosa hypersusceptible mutants. Error bars indicate standard deviations of the results from four independent experiments. Statistically significant differences (P < 0.005) in the survival of nematodes with respect to the wild-type strain were evaluated using Student's t test and are highlighted with two asterisks.
DISCUSSION
Within this work, we define new members of the P. aeruginosa intrinsic resistome and new potential mechanisms for mutation-driven acquisition of resistance to aminoglycosides. In addition to genes already known to be involved in P. aeruginosa intrinsic resistance to aminoglycosides, we identified two novel loci (glnD and mucD) in the chromosome of P. aeruginosa PA14 that contribute to intrinsic resistance to at least one aminoglycoside (see Table S1 in the supplemental material), although inactivation of mucD increases susceptibility to one aminoglycoside and reduces susceptibility to another. Interestingly, both genes have been proposed to play a potential role in β-lactam resistance (26, 36). Further, 14 novel loci could potentially be involved in the acquisition of mutational resistance to at least one aminoglycoside, since their inactivation increases the MICs of such aminoglycosides by at least 3-fold compared with the wild-type parental strain. These loci are PA5183, waaL, PA1440, PA3844, PA4874, PA1411, pilC, PA3350, nppB, flgH, flgI, nosR, ppsA, and purF (Table S1). It is worth mentioning that pilC mutant was one of the two mutants that showed a 3-fold MIC increase associated with the four tested aminoglycosides. To date, this gene required for the biogenesis of the P. aeruginosa pili has not been reported to be involved in antibiotic resistance. The higher resistance of the mutant could be explained by changes in the membrane potential and permeability due to an anomalous pilus structure, which would affect aminoglycoside uptake.
Note here that mutation-driven resistance is mainly relevant in the case of chronic infections and that resistance to aminoglycosides is frequently due to the acquisition of inactivating enzymes, a feature not analyzed in the current work.
Notably, only a few mutants exhibited simultaneous susceptibility changes with respect to the aminoglycosides included in our study (Fig. 1). This indicates that, while these antibiotics share a mechanism of action, the potential mechanisms of acquiring resistance due to gene inactivation and the elements contributing to intrinsic resistance are not necessarily the same for each of these antibiotics. These results support the idea that, at least in the case of aminoglycosides, the role of a particular gene in the resistome of a given antibiotic cannot be generalized to all members within its family.
The genes whose inactivation increases the susceptibility to antibiotics are suitable targets in the search of coadjuvants able to sensitize bacteria to such elements. Hence, we focused our studies on those mutants that exhibited at least a 3-fold increase in aminoglycosides' susceptibility. In addition to an increase in susceptibility to antibiotics from other structural families, these chosen mutants presented defective phenotypes in biofilm formation, elastase activity, and swarming motility. The PA4463 mutant has increased susceptibility to aminoglycosides and to tetracycline. The mutant is also impaired in biofilm formation and in the production of elastase. PA4463 (hpf) codes for a hibernation-promoting factor that is required for rRNA preservation under prolonged nutrient starvation conditions, including the dormant state of certain subpopulations that are present in P. aeruginosa biofilms (31, 32). Interestingly, these biofilms have been shown to tolerate the antibiotics ceftazidime and tobramycin at levels far greater than those necessary to eliminate planktonic bacteria (37, 38). In fact, it is believed that this phenomenon may be due in part to the persistent subpopulations within the biofilms mentioned above, which are able to repopulate them when the treatment finishes (39, 40).
Concerning hflK (PA4942) and PA2798, their role in aminoglycoside resistance has been described previously by Krahn et al. (16) and Hinz et al. (29), respectively. Our results show that, in addition, these mutants are more susceptible to various antimicrobials, in particular, tigecycline, tetracycline, fosfomycin, erythromycin (PA2798), and aztreonam and fosfomycin (hflK), and are impaired in the production of virulence determinants. The fact that PA2798 codes for a two-component regulator might be the reason for the effects observed in almost every tested phenotype. Actually, coregulation of biofilm formation, elastase activity, and swimming motility by another common regulator has been recently described in P. aeruginosa (41), suggesting these processes to be interconnected. Conversely, HflK is one subunit of the inner membrane protein complex HflKC, which participates in quality control of integral membrane and cytosolic proteins (42). Consequently, one possible cause of the phenotype shown by the mutant lacking this protein may be represented by the pleiotropic effects of the alteration in the complex network of proteases to which HflK belongs, which seems to affect the susceptibility to several classes of antibiotics and to other stressors such as alkaline pH and other compounds (29).
Finally, the PA3658 mutant exhibits increased susceptibility to aminoglycosides, tigecycline, tetracycline, ciprofloxacin, and fosfomycin. In addition, it displays a less lethal action against C. elegans, produces less pyocyanin than P. aeruginosa PA14, and is strongly impaired in the development of such phenotypes with relevance for infection as biofilm formation, elastase activity, and swarming motility. Thus, inactivation of glnD (PA3658) results in nonvirulent and nonresistant behavior, although the mechanisms behind this phenotype remain to be established. Note the increased susceptibility to tigecycline and fosfomycin that this mutant showed, because P. aeruginosa is intrinsically resistant to tigecycline (43); whereas fosfomycin is one of the antibiotics of choice for the treatment of P. aeruginosa infections (4). Very little is known about glnD in P. aeruginosa, aside from the fact that it encodes an uridylyltransferase associated with the glycine betaine catabolism (44) and its relationship with N2 catabolism in other bacteria (28). Therefore, further research is needed for understanding the molecular basis of the increased susceptibility to antibiotics and reduced virulence displayed by P. aeruginosa when glnD is inactivated.
Our results allow the identification of genes that are likely to be simultaneously involved in intrinsic antibiotic resistance and virulence of P. aeruginosa. This may unlock new ways of managing and treating infections of this pathogen; for instance, targeting the locus PA3658 with a proper inhibitor might prevent P. aeruginosa from developing a virulent behavior and a resistance phenotype against clinically important antibiotics, such as tobramycin, amikacin, and fosfomycin, or might even dissipate its intrinsic resistance to tigecycline, which would lead us to reconsider the clinical use of this antibiotic upon GlnD inhibition.
MATERIALS AND METHODS
Identification of mutants with altered susceptibility to amikacin.
The screening was performed using an agar dilution method as described previously (15, 26). A nonredundant transposon insertion library of P. aeruginosa PA14 (25) harboring 5,850 mutations representing 4,596 genes was used for the screening. PCR amplification was used to verify the presence of the transposon MAR2xT7 in the inactivated genes of five selected hypersusceptible mutants. Five primer pairs, which amplified 150-to-300-bp regions surrounding the transposon in each of the analyzed mutants, were designed (see Table S2 in the supplemental material). After PCR amplification, the sizes of the corresponding amplicons were assessed in comparison to the amplifications in P. aeruginosa PA14 strain in a 1% agarose gel.
Analysis of susceptibility to antibiotics.
The susceptibility to amikacin, tobramycin, kanamycin, and streptomycin in all selected mutants and to tigecycline, tetracycline, aztreonam, ceftazidime, imipenem, ciprofloxacin, erythromycin, chloramphenicol, and fosfomycin in a subset of strains was determined using MIC strips (MIC Test Strip; Liofilchem) in Mueller-Hinton agar (MHA) (Sigma) at 37°C. The mutants were grouped as a function of their aminoglycoside MICs using Gene Cluster 3.0 software. The hierarchical cluster was displayed using Java Treeview software. MICs were normalized to the value of the wild-type strain using the formula log2 [MICmutant/MICPA14].
Elastase activity and pyocyanin production.
The different bacterial strains were cultured at 37°C in 10 ml of LB broth. After 24 h of culture, 1-ml samples were collected and centrifuged for 10 min at 7,000 rpm, and the supernatants were filtered through 0.2-μm-pore-size filters (Whatman). The elastase assay was adapted from a method previously described by Kessler and Safrin (45) as follows: 1 ml of Congo red elastin (Sigma-Aldrich) was added to 100 μl of each sample, and the mixture was incubated at 37°C and 250 rpm for 2 h. Subsequently, samples were centrifuged (10 min, 7,000 rpm) and the optical density at 495 nm (OD495) of 100 μl of the filtered supernatants was determined using a 96-well microtiter plate (Nunc) in a Tecan Infinite M200 plate reader (Tecan). Pyocyanin production was determined by measuring the OD690 of 100 μl of filtered supernatants using the same plate reader. Three replicates of each strain were included in the analyses.
Biofilm formation.
Biofilm formation was tested using 96-well microtiter plates (Falcon 3911 Microtest III flexible assay plate) previously sterilized with UV light. A modification of a previously reported protocol (46) was followed. A 1:100 dilution of overnight LB broth bacterial cultures was inoculated into the microtiter plate (100 μl/well) and incubated at 37°C for 48 h. Next, 25 μl of a 0.1% crystal violet solution was added to each well (5 min), and the excess dye was repeatedly and thoroughly rinsed with distilled water (4 times). Triton X-100 (0.25%) was added to detach the biofilm from the wells, and 100 μl of each sample was transferred to a 96-well microtiter plate (Nunc). The biofilm quantification was performed by measuring the OD570 in a Tecan Infinite M200 plate reader (Tecan). Eight replicates of each strain were included in the assay.
Swarming assay.
Swarming assays were performed in petri dishes with 25 ml of a Casamino Acids medium that contained 0.5% Casamino Acids, 0.5% Bacto agar, 0.5% filtered glucose, 3.3 mM K2HPO4, and 3 mM MgSO4. A 4-μl inoculum (OD600 of 1) of either P. aeruginosa PA14 or one of the mutant strains was placed on the center of the agar surface. Three replicates of each strain were incubated for 17 h at 37°C. The diameter of the swarming motility zone was measured and a picture was recorded of every plate.
Caenorhabditis elegans virulence assay.
The kinetics of C. elegans killing by P. aeruginosa PA14 and its derivatives was assessed by using the method previously described by Tan et al. (47), with some modifications. A 50-μl inoculum from each strain (four replicates of each) was grown in 6-cm-diameter plates with potato dextrose agar (PDA; Sigma-Aldrich) for 24 h at 37°C, in order to form a bacterial lawn. Each plate was subsequently seeded with 5 L4-stage hermaphrodite C. elegans N2 Bristol worms (48), and plates were incubated at 18°C for a week. Plates were examined for living worms every day during this period. A worm was considered dead when it no longer responded to touch. E. coli OP50 was used as a positive control of the preferred food source known to have reduced virulence in P. aeruginosa.
Supplementary Material
ACKNOWLEDGMENTS
Work in our laboratory is supported by grants from Instituto de Salud Carlos III (Spanish Network for Research on Infectious Diseases [RD16/0016/0011], cofinanced by European Development Regional Fund “A way to achieve Europe” [ERDF]), from the Spanish Ministry of Economy and Competitivity (BIO2017-83128-R), and from the Autonomous Community of Madrid (B2017/BMD-3691). F.S.-G. is the recipient of an FPU fellowship from the Spanish Ministry of Education.
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/AAC.00185-19.
REFERENCES
- 1.Silby MW, Winstanley C, Godfrey SA, Levy SB, Jackson RW. 2011. Pseudomonas genomes: diverse and adaptable. FEMS Microbiol Rev 35:652–680. doi: 10.1111/j.1574-6976.2011.00269.x. [DOI] [PubMed] [Google Scholar]
- 2.Tummler B, Wiehlmann L, Klockgether J, Cramer N. 2014. Advances in understanding Pseudomonas. F1000Prime Rep 6:9. doi: 10.12703/P6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martinez-Solano L, Macia MD, Fajardo A, Oliver A, Martinez JL. 2008. Chronic Pseudomonas aeruginosa infection in chronic obstructive pulmonary disease. Clin Infect Dis 47:1526–1533. doi: 10.1086/593186. [DOI] [PubMed] [Google Scholar]
- 4.Bassetti M, Vena A, Croxatto A, Righi E, Guery B. 2018. How to manage Pseudomonas aeruginosa infections. Drugs Context 7:212527. doi: 10.7573/dic.212527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mena A, Smith EE, Burns JL, Speert DP, Moskowitz SM, Perez JL, Oliver A. 2008. Genetic adaptation of Pseudomonas aeruginosa to the airways of cystic fibrosis patients is catalyzed by hypermutation. J Bacteriol 190:7910–7917. doi: 10.1128/JB.01147-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cantón R, Cobos N, de Gracia J, Baquero F, Honorato J, Gartner S, Alvarez A, Salcedo A, Oliver A, García-Quetglas E. 2005. Antimicrobial therapy for pulmonary pathogenic colonisation and infection by Pseudomonas aeruginosa in cystic fibrosis patients. Clin Microbiol Infect 11:690–703. doi: 10.1111/j.1469-0691.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 7.Holbrook SYL, Garneau-Tsodikova S. 2018. Evaluation of aminoglycoside and carbapenem resistance in a collection of drug-resistant Pseudomonas aeruginosa clinical isolates. Microb Drug Resist 24:1020–1030. doi: 10.1089/mdr.2017.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Merlo CA, Boyle MP, Diener-West M, Marshall BC, Goss CH, Lechtzin N. 2007. Incidence and risk factors for multiple antibiotic-resistant Pseudomonas aeruginosa in cystic fibrosis. Chest 132:562–568. doi: 10.1378/chest.06-2888. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt O, Lehmann C, Madabushi R, Kumar V, Derendorf H, Welte T. 2006. Once-daily tobramycin in cystic fibrosis: better for clinical outcome than thrice-daily tobramycin but more resistance development? J Antimicrob Chemother 58:822–829. doi: 10.1093/jac/dkl328. [DOI] [PubMed] [Google Scholar]
- 10.Geller DE. 2009. Aerosol antibiotics in cystic fibrosis. Respir Care 54:658–670. doi: 10.4187/aarc0537. [DOI] [PubMed] [Google Scholar]
- 11.Gomez MJ, Neyfakh AA. 2006. Genes involved in intrinsic antibiotic resistance of Acinetobacter baylyi. Antimicrob Agents Chemother 50:3562–3567. doi: 10.1128/AAC.00579-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez JL, Baquero F, Andersson DI. 2007. Predicting antibiotic resistance. Nat Rev Microbiol 5:958–965. doi: 10.1038/nrmicro1796. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock REW, Martínez JL. 2011. The intrinsic resistome of Pseudomonas aeruginosa to beta-lactams. Virulence 2:144–146. doi: 10.4161/viru.2.2.15014. [DOI] [PubMed] [Google Scholar]
- 14.Breidenstein EB, Khaira BK, Wiegand I, Overhage J, Hancock RE. 2008. Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob Agents Chemother 52:4486–4491. doi: 10.1128/AAC.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fernández L, Alvarez-Ortega C, Wiegand I, Olivares J, Kocíncová D, Lam JS, Martínez JL, Hancock REW. 2013. Characterization of the polymyxin B resistome of Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:110–119. doi: 10.1128/AAC.01583-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krahn T, Gilmour C, Tilak J, Fraud S, Kerr N, Lau CH, Poole K. 2012. Determinants of intrinsic aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 56:5591–5602. doi: 10.1128/AAC.01446-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fajardo A, Martínez-Martín N, Mercadillo M, Galán JC, Ghysels B, Matthijs S, Cornelis P, Wiehlmann L, Tümmler B, Baquero F, Martínez JL. 2008. The neglected intrinsic resistome of bacterial pathogens. PLoS One 3:e1619. doi: 10.1371/journal.pone.0001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schurek KN, Marr AK, Taylor PK, Wiegand I, Semenec L, Khaira BK, Hancock RE. 2008. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:4213–4219. doi: 10.1128/AAC.00507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vestergaard M, Leng B, Haaber J, Bojer MS, Vegge CS, Ingmer H. 2016. Genome-wide identification of antimicrobial intrinsic resistance determinants in Staphylococcus aureus. Front Microbiol 7:2018. doi: 10.3389/fmicb.2016.02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu A, Tran L, Becket E, Lee K, Chinn L, Park E, Tran K, Miller JH. 2010. Antibiotic sensitivity profiles determined with an Escherichia coli gene knockout collection: generating an antibiotic bar code. Antimicrob Agents Chemother 54:1393–1403. doi: 10.1128/AAC.00906-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brannon JR, Hadjifrangiskou M. 2016. The arsenal of pathogens and antivirulence therapeutic strategies for disarming them. Drug Des Devel Ther 10:1795–1806. doi: 10.2147/DDDT.S98939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Williams P. 2002. Quorum sensing: an emerging target for antibacterial chemotherapy? Expert Opin Ther Targets 6:257–274. doi: 10.1517/14728222.6.3.257. [DOI] [PubMed] [Google Scholar]
- 23.Martinez JL, Baquero F. 2002. Interactions among strategies associated with bacterial infection: pathogenicity, epidemicity, and antibiotic resistance. Clin Microbiol Rev 15:647–679. doi: 10.1128/CMR.15.4.647-679.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guo Q, Wei Y, Xia B, Jin Y, Liu C, Pan X, Shi J, Zhu F, Li J, Qian L, Liu X, Cheng Z, Jin S, Lin J, Wu W. 2016. Identification of a small molecule that simultaneously suppresses virulence and antibiotic resistance of Pseudomonas aeruginosa. Sci Rep 6:19141. doi: 10.1038/srep19141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock REW, Martínez JL. 2010. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to beta-lactam antibiotics. Antimicrob Agents Chemother 54:4159–4167. doi: 10.1128/AAC.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sun E, Gill EE, Falsafi R, Yeung A, Liu S, Hancock REW. 27 August 2018. Broad-spectrum adaptive antibiotic resistance associated with Pseudomonas aeruginosa mucin-dependent surfing motility. Antimicrob Agents Chemother doi: 10.1128/AAC.00848-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Contreras A, Drummond M, Bali A, Blanco G, Garcia E, Bush G, Kennedy C, Merrick M. 1991. The product of the nitrogen fixation regulatory gene nfrX of Azotobacter vinelandii is functionally and structurally homologous to the uridylyltransferase encoded by glnD in enteric bacteria. J Bacteriol 173:7741–7749. doi: 10.1128/jb.173.24.7741-7749.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hinz A, Lee S, Jacoby K, Manoil C. 2011. Membrane proteases and aminoglycoside antibiotic resistance. J Bacteriol 193:4790–4797. doi: 10.1128/JB.05133-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee S, Hinz A, Bauerle E, Angermeyer A, Juhaszova K, Kaneko Y, Singh PK, Manoil C. 2009. Targeting a bacterial stress response to enhance antibiotic action. Proc Natl Acad Sci U S A 106:14570–14575. doi: 10.1073/pnas.0903619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Akiyama T, Williamson KS, Schaefer R, Pratt S, Chang CB, Franklin MJ. 2017. Resuscitation of Pseudomonas aeruginosa from dormancy requires hibernation promoting factor (PA4463) for ribosome preservation. Proc Natl Acad Sci U S A 114:3204–3209. doi: 10.1073/pnas.1700695114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williamson KS, Richards LA, Perez-Osorio AC, Pitts B, McInnerney K, Stewart PS, Franklin MJ. 2012. Heterogeneity in Pseudomonas aeruginosa biofilms includes expression of ribosome hibernation factors in the antibiotic-tolerant subpopulation and hypoxia-induced stress response in the metabolically active population. J Bacteriol 194:2062–2073. doi: 10.1128/JB.00022-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lau CH, Fraud S, Jones M, Peterson SN, Poole K. 2013. Mutational activation of the AmgRS two-component system in aminoglycoside-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 57:2243–2251. doi: 10.1128/AAC.00170-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greipel L, Fischer S, Klockgether J, Dorda M, Mielke S, Wiehlmann L, Cramer N, Tummler B. 2016. Molecular epidemiology of mutations in antimicrobial resistance loci of Pseudomonas aeruginosa isolates from airways of cystic fibrosis patients. Antimicrob Agents Chemother 60:6726–6734. doi: 10.1128/AAC.00724-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lau CH, Krahn T, Gilmour C, Mullen E, Poole K. 2015. AmgRS-mediated envelope stress-inducible expression of the mexXY multidrug efflux operon of Pseudomonas aeruginosa. Microbiologyopen 4:121–135. doi: 10.1002/mbo3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sanz-Garcia F, Hernando-Amado S, Martinez JL. 24 September 2018. Mutation-driven evolution of Pseudomonas aeruginosa in the presence of either ceftazidime or ceftazidime-avibactam. Antimicrob Agents Chemother doi: 10.1128/AAC.01379-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nickel JC, Ruseska I, Wright JB, Costerton JW. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother 27:619–624. doi: 10.1128/AAC.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anwar H, Costerton JW. 1990. Enhanced activity of combination of tobramycin and piperacillin for eradication of sessile biofilm cells of Pseudomonas aeruginosa. Antimicrob Agents Chemother 34:1666–1671. doi: 10.1128/AAC.34.9.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulcahy LR, Burns JL, Lory S, Lewis K. 2010. Emergence of Pseudomonas aeruginosa strains producing high levels of persister cells in patients with cystic fibrosis. J Bacteriol 192:6191–6199. doi: 10.1128/JB.01651-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lewis K. 2007. Persister cells, dormancy and infectious disease. Nat Rev Microbiol 5:48–56. doi: 10.1038/nrmicro1557. [DOI] [PubMed] [Google Scholar]
- 41.Li Y, Bai F, Xia H, Zhuang L, Xu H, Jin Y, Zhang X, Bai Y, Qiao M. 2015. A novel regulator PA5022 (aefA) is involved in swimming motility, biofilm formation and elastase activity of Pseudomonas aeruginosa. Microbiol Res 176:14–20. doi: 10.1016/j.micres.2015.04.001. [DOI] [PubMed] [Google Scholar]
- 42.Kihara A, Akiyama Y, Ito K. 1996. A protease complex in the Escherichia coli plasma membrane: HflKC (HflA) forms a complex with FtsH (HflB), regulating its proteolytic activity against SecY. EMBO J 15:6122–6131. doi: 10.1002/j.1460-2075.1996.tb01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martinez JL, Coque TM, Baquero F. 2015. What is a resistance gene? Ranking risk in resistomes. Nat Rev Microbiol 13:116–123. doi: 10.1038/nrmicro3399. [DOI] [PubMed] [Google Scholar]
- 44.Wargo MJ, Szwergold BS, Hogan DA. 2008. Identification of two gene clusters and a transcriptional regulator required for Pseudomonas aeruginosa glycine betaine catabolism. J Bacteriol 190:2690–2699. doi: 10.1128/JB.01393-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kessler E, Safrin M. 2014. Elastinolytic and proteolytic enzymes. Methods Mol Biol 1149:135–169. doi: 10.1007/978-1-4939-0473-0_13. [DOI] [PubMed] [Google Scholar]
- 46.O'Toole GA, Kolter R. 1998. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol 28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 47.Tan MW, Rahme LG, Sternberg JA, Tompkins RG, Ausubel FM. 1999. Pseudomonas aeruginosa killing of Caenorhabditis elegans used to identify P. aeruginosa virulence factors. Proc Natl Acad Sci U S A 96:2408–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brenner S. 1974. The genetics of Caenorhabditis elegans. Genetics 77:71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.