Identifying and understanding potential drug-drug interactions (DDIs) are vital for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. This article discusses DDIs between doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), and cytochrome P450 3A (CYP3A) substrates and drugs that modulate CYP3A activity.
KEYWORDS: HIV, doravirine, drug-drug interactions, nonnucleoside reverse transcriptase inhibitor (NNRTI)
ABSTRACT
Identifying and understanding potential drug-drug interactions (DDIs) are vital for the treatment of human immunodeficiency virus type 1 (HIV-1) infection. This article discusses DDIs between doravirine, a nonnucleoside reverse transcriptase inhibitor (NNRTI), and cytochrome P450 3A (CYP3A) substrates and drugs that modulate CYP3A activity. Consistent with previously published in vitro data and DDI trials with the CYP3A substrates midazolam and atorvastatin, doravirine did not have any meaningful impact on the pharmacokinetics of the CYP3A substrates ethinyl estradiol and levonorgestrel. Coadministration of doravirine with CYP3A inhibitors (ritonavir or ketoconazole) increased doravirine exposure approximately 3-fold. However, these increases were not considered clinically meaningful. Conversely, previously published trials showed that coadministered CYP3A inducers (rifampin and rifabutin) decreased doravirine exposure by 88% and 50%, respectively (K. L. Yee, S. G. Khalilieh, R. I. Sanchez, R. Liu, et al., Clin Drug Investig 37:659–667, 2017 [https://doi.org/10.1007/s40261-017-0513-4]; S. G. Khalilieh, K. L. Yee, R. I. Sanchez, R. Liu, et al., J Clin Pharmacol 58:1044–1052, 2018 [https://doi.org/10.1002/jcph.1103]), while doravirine exposure following prior efavirenz administration led to an initial reduction in doravirine exposure of 62%, but the reduction became less pronounced with time (K. L. Yee, R. I. Sanchez, P. Auger, R. Liu, et al., Antimicrob Agents Chemother 61:e01757-16, 2017 [https://doi.org/10.1128/AAC.01757-16]). Overall, the coadministration of doravirine with CYP3A inhibitors and substrates is, therefore, supported by these data together with efficacy and safety data from clinical trials, while coadministration with strong CYP3A inducers, such as rifampin, cannot be recommended. Concomitant dosing with rifabutin (a CYP3A inducer less potent than rifampin) is acceptable if doravirine dosing is adjusted from once to twice daily; however, the effect of other moderate inducers on doravirine pharmacokinetics is unknown.
INTRODUCTION
Human immunodeficiency virus type 1 (HIV-1) infection is a chronic disease associated with diverse comorbidities (1, 2). As the HIV-1-infected population ages, the prevalence of comorbidities and required treatments increases (3, 4). Comorbidities that are more common in older HIV-infected adults include hypertension, hypercholesterolemia, diabetes mellitus, and renal disease (4). Therefore, polypharmacy is common among individuals with HIV-1 infection, especially for older individuals (5). However, polypharmacy is not limited to the treatment of age-associated comorbidities and is an important consideration for people of all ages being treated for HIV-1 infection. For example, women of childbearing age may choose to receive concomitant oral contraceptive therapy to prevent pregnancy (6). Tuberculosis (7) and hepatitis C virus infection (8) have also emerged as significant non-age-related comorbidities. Furthermore, the current HIV-1 treatment guidelines generally require the use of three active treatments from two or more drug classes (9). This requirement means that individuals being treated for HIV-1 infection concurrently take multiple medications; therefore, it is important to determine potential drug-drug interaction (DDI) profiles between these medications.
Doravirine (MK-1439) is a novel nonnucleoside reverse transcriptase inhibitor (NNRTI) (10) for the treatment of HIV-1 infection in combination with other antiretroviral therapies (ARTs). In the United States, doravirine is approved for the treatment of HIV-1 infection in adult patients with no prior ART history in combination with other antiretroviral agents and as a three-drug combination with lamivudine and tenofovir disoproxil fumarate as a complete regimen (11, 12). Doravirine is generally well tolerated with durable efficacy (13, 14) and is active in vitro against wild-type and common NNRTI-resistant HIV-1 strains (15). The approved clinical dose of 100 mg once daily is coadministered with existing ARTs to people living with HIV (11, 12) and is expected to be administered alongside a variety of treatments for comorbid conditions.
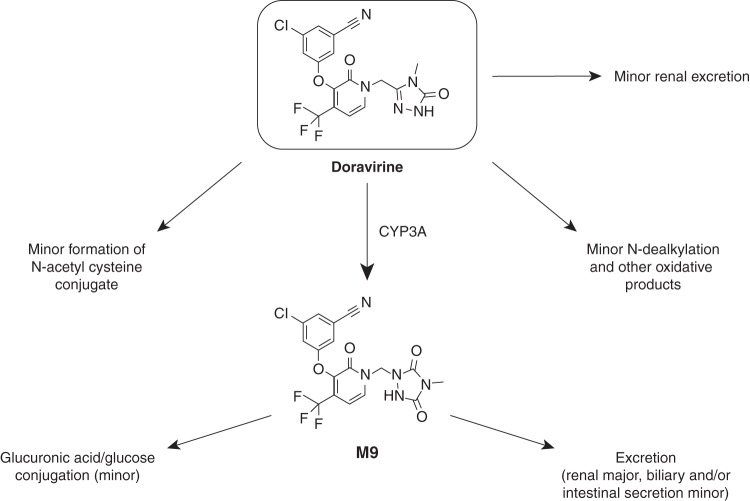
Metabolic profile of doravirine.
Previous studies have established the metabolic profile of doravirine (Fig. 1). In vitro, doravirine is metabolized via cytochrome P450 3A (CYP3A)-mediated oxidation (16), leading to the potential for doravirine metabolism to be affected in the presence of CYP3A inhibitors or inducers. Metabolism was shown to be mediated primarily by CYP3A4, with a smaller contribution by CYP3A5; doravirine metabolism was almost completely inhibited by anti-CYP3A antibodies, and no metabolism was observed upon incubation with other cytochrome P450 (CYP) enzymes (16). Consistent with these data, in a human absorption, metabolism, and excretion (AME) trial, oxidative metabolism was the predominant route of elimination for [14C]doravirine, while renal excretion was a minor elimination pathway (16). Doravirine is also a substrate for human P-glycoprotein (P-gp) in vitro; however, the high apparent permeation of doravirine, ∼25 × 10−6 cm/s, makes interactions with P-gp inhibitors unlikely (16). In vitro studies showed that at clinically relevant concentrations, doravirine was not an inhibitor of major CYP enzymes or uridine 5′-diphospho-glucuronosyltransferase 1A1 (UGT1A1) and was not a meaningful inducer of CYP1A2, CYP2B6, or CYP3A4 (17). Additionally, doravirine was considered to have a low potential for interaction with substrates of organic anion-transporting polypeptide 1B1 (OATP1B1), OATP1B3, organic anion transporter 1 (OAT1), OAT3, organic cation transporter 2 (OCT2), or breast cancer resistance protein (BCRP) (17). Based on its limited renal elimination (10, 16), doravirine is not expected to inhibit other renal transporters, such as multidrug and toxin extrusion protein 1/2K. These data suggest that doravirine is unlikely to cause clinically relevant DDIs; however, doravirine could be affected by the modulation of CYP3A activity by other drugs.
FIG 1.
Elimination pathways of doravirine in humans.
Evaluation of DDIs between doravirine and concomitant medications that are substrates for or modulators of CYP3A.
A number of previous reports have investigated the potential DDIs of doravirine with concomitant medications, including rifampin (18), rifabutin (19), efavirenz (20), midazolam (10), and atorvastatin (21). This article expands on these previous trials to report the findings of three additional DDI trials of doravirine with ritonavir, ketoconazole, and ethinyl estradiol (EE)-levonorgestrel (LNG), drugs known to have effects or dependencies on CYP3A, the primary enzyme involved in doravirine metabolism. These findings are discussed in the context of the previous reports of CYP3A-mediated DDI trials of doravirine (10, 18–21) in order to evaluate doravirine both as a perpetrator and as a victim of CYP3A interactions (causing and being affected by DDIs, respectively). The studied drugs and the rationale for their investigation are briefly introduced below.
(i) CYP3A substrate: oral contraceptive EE-LNG.
Women and female adolescents accounted for 19% of the nearly 40,000 new HIV infections in the United States in 2015 (22). Therefore, females of reproductive potential are an important segment of the HIV-1-infected clinical population to which doravirine is targeted; as a result, oral contraceptives are a key concomitant medication for this population. The ethinyl estradiol-levonorgestrel (EE-LNG; 0.03 mg/0.15 mg) combination is a fixed-dose oral contraceptive pill that is widely available and that has a large worldwide market. EE and LNG are metabolized by a number of enzymes involving both hydroxylation and conjugation (23–25), and both are substrates of CYP3A (24, 26).
(ii) CYP3A inhibitor: ritonavir.
Ritonavir is an antiretroviral protease inhibitor with a complex drug interaction profile due to its potential to inhibit and/or induce multiple drug-metabolizing enzymes and transporters (27). Depending on the contribution of different enzymes and transporters to the disposition of a drug, ritonavir coadministration may result in inhibition or induction of various magnitudes; however, the net effect of ritonavir on drugs that are eliminated predominantly by CYP3A is an increase in plasma concentrations, as CYP3A inactivation predominates, despite increases in enzyme levels (27). Thus, ritonavir is primarily used as a pharmacokinetic (PK)-enhancing agent (booster) for other ARTs through inhibition of CYP3A (9, 28, 29). Ritonavir-boosted darunavir forms part of the protease inhibitor-based regimen recommended in certain clinical situations in the United States for treatment-naive people living with HIV, and other ritonavir-boosted protease inhibitor regimens may be used if initial treatment fails (9). As well as inhibiting CYP3A, ritonavir is a potential mixed inducer/inhibitor of P-gp (30) and is also thought to induce CYP1A2/2C9/2C19 (31) and UGT1A1/1A3/1A4 (32).
(iii) CYP3A inhibitor: ketoconazole.
Ketoconazole is a strong CYP3A inhibitor (33) that also demonstrates autoinhibition after multiple doses (34) and, as such, is commonly used in DDI studies to probe the effect of CYP3A inhibition (35). Ketoconazole is an antifungal agent that can be used for treating opportunistic infections (34), which often occur in people living with HIV (36), although its current use is limited (37). Other antifungals, such as itraconazole, voriconazole, and fluconazole, are also CYP3A inhibitors, and, therefore, the results of this study are relevant to more commonly used antifungals. Ketoconazole is also a strong inhibitor of P-gp (38) and UGT1A isoforms (33, 39).
RESULTS
Effect of doravirine on single-dose EE-LNG (oral contraceptive) PK.
Twenty eligible women were enrolled, and 19 of them completed the trial (Table 1). The baseline demographic characteristics are shown in Table 2. Summary statistics for EE and LNG plasma PK are shown in Table 3. The area under the concentration-time curve from time zero extrapolated to infinity (AUC0–∞) values for EE were comparable between coadministration of EE-LNG and doravirine and administration of EE-LNG alone and were increased slightly (21%) for LNG during coadministration. The maximum plasma concentration (Cmax) for EE was lower (17%) when EE-LNG was administered alongside multiple doses of doravirine than when EE-LNG was administered alone, whereas for LNG, the Cmax values were similar with and without coadministration of doravirine. A single dose of EE-LNG coadministered with multiple doses of doravirine was generally well tolerated. Twelve participants (60%) reported 27 clinical adverse events (AEs), the most common of which were rhinorrhea (three participants, 15%), irritability (two participants, 10%), and diarrhea (two participants, 10%). No other AE was reported by more than one participant. Three participants (15%) reported three AEs that were deemed to be drug related, and each AE was reported by only one participant (5%). Two of these drug-related AEs were deemed to be related to doravirine (oral herpes and erythematous rash), and one was related to the coadministration of doravirine and EE-LNG (nervousness). One participant (5%) had a laboratory AE (red blood cells in urine) that was considered related to the coadministration of EE-LNG with doravirine. One participant (5%) discontinued prior to dosing on day 4 of period 2 due to an AE (elevated blood pressure) which was not considered related to treatment. All AEs had resolved by the end of the trial, and no serious AEs (SAEs) or deaths were reported. No consistent treatment-related differences in laboratory tests, vital signs, or electrocardiograms were observed.
TABLE 1.
Trial design: doravirine drug interaction trials with EE-LNG, ritonavir, and ketoconazolea
| Trial | Trial design and description | Key inclusion and exclusion criteria |
Treatment |
Blood sampling |
||||
|---|---|---|---|---|---|---|---|---|
| Inclusion criteria | Exclusion criteria | Period 1 | Period 2 | General comments | Period 1 | Period 2 | ||
| CYP3A substrate: effects of multiple-dose doravirine on single-dose PK of an oral contraceptive, EE-LNG | Phase 1, open-label, two-period, fixed-sequence trial (protocol no. MK-1439-012); trial dates, 1 February 2013 to 1 April 2013; 20 women were enrolled, and 19 women completed the trial | Healthy women aged 18–65 yr; postmenopausal or oophorectomized; BMI = 18.5–32.0 kg/m2 | Positive result for HIV, hepatitis B virus surface antigen, or hepatitis C virus infection; use of any drugs or substances known to be inducers of CYP enzymes and/or P-gp, including St. John’s wort, within 28 days or 5 times the half-life of the product (whichever was longer) prior to the first dose of trial drug or known to be significant inhibitors of CYP enzymes and/or significant inhibitors or substrates of P-gp, OATP, UGT, and/or SULT1E1 within 14 days or 5 times the half-life of the trial drug (whichever was longer) prior to the first dose of trial drug | Single dose of EE at 0.03 mg and LNG at 0.15 mg on day 1, followed by a 7-day washout period | Doravirine was administered at 100 mg QD on days 1–17 and was coadministered with EE at 0.03 mg and LNG at 0.15 mg on day 14 | EE-LNG was administered in a fasted state (≥10 h predose and ≥4 h postdose); doravirine was administered in a fasted state (≥1 h predose and ≥2 h postdose), except on day 14, when it was coadministered with EE-LNG in the fasting state (≥10 h predose and ≥4 h postdose) | For samples for EE and LNG assay, predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h postdose | For samples for EE and LNG assay, on day 14, predose and at 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 24, 48, 72, and 96 h postdose |
| CYP3A inhibitor: effects of multiple-dose ritonavir on single-dose PK of doravirine | Phase 1, open-label, fixed-sequence, two-period trial (protocol number MK-1439-002, EudraCT no. 2011-002722-48); trial dates, 16 August 2011 to 16 November 2011; 8 men were enrolled, and 8 men completed the trial | Healthy men aged between 18 and 50 yr; BMI ≤ 35 kg/m2 | History of documented HIV infection; concomitant use of medications or herbal remedies beginning approximately 2 wk (or 5 half-lives) prior to initial dose of trial drug until the posttrial visit | Single dose of doravirine 50 mg (day 1), followed by a 7-day washout period | Ritonavir was administered at 100 mg twice daily on days 1–20 and was coadministered with a single dose of doravirine at 50 mg on the morning of day 14 | Doravirine was administered in a fasted state (fasting for ≥8 h predose and 4 h postdose); ritonavir was administered within 30 min prior to or after a meal, except on day 14, when it was coadministered with doravirine in a fasted state (fasting ≥8 h predose and 4 h postdose) | Predose and at 0.5, 1, 2, 3, 4, 5, 6, 8 10, 12, 16, 24, 48, 72, 96, and 120 h postdose | Predose and at 0.5, 1, 2, 3, 4, 5, 6, 8 10, 12, 16, 24, 48, 72, 96, 120, 144, and 168 h postdose (day 14) |
| CYP3A inhibitor: effects of multiple-dose ketoconazole on single-dose PK of doravirine | Phase 1, open-label, two-period, fixed-sequence trial (protocol no. MK-1439-010; trial dates, 21 November 2012 to 31 December 2012; 8 men and 2 women were enrolled, and 8 men and 2 women completed the trial | Healthy men and women between 19 and 50 yr of age; women were required to be of nonchildbearing potential; BMI = 18.5–32.0 kg/m2 | Positive result for HIV, hepatitis B virus surface antigen, or hepatitis C virus infection; use of any drugs or substances known to be significant inhibitors or inducers of CYP enzymes, significant inhibitors, inducers, or substrates of P-gp, or significant inhibitors or substrates of OATP within 14 days (for inhibitors/substrates) or 28 days (for inducers) or within 5 half-lives before the first trial drug dose | Single dose of doravirine 100 mg (day 1), followed by a ≥7-day washout period | Ketoconazole was administered at 400 mg QD on days 1–10 and was coadministered with doravirine at 100 mg on the morning of day 2 | Doravirine was administered in a fasted state (fasting for ≥10 h predose and 4 h postdose); ketoconazole was administered in a fasted state (fasting for ≥1 h predose and ≥2 h postdose), except on day 2, when it was administered with doravirine in a fasted state (fasting for ≥10 h predose and 4 h postdose) | Predose and at 0.5, 1, 1.5, 2, 3, 6, 12, 24, 30, 48, and 72 h postdose |
On day 2, predose and at 1, 1.5, 2, 3, 6, 12, 24, 48, 96, 144, and 216 h postdose |
BMI, body mass index; CYP3A, cytochrome P450 3A; EE, ethinyl estradiol; HIV, human immunodeficiency virus; LNG, levonorgestrel; OATP, organic-anion-transporting polypeptide; PK, pharmacokinetic; QD, once daily.
TABLE 2.
Trial population disposition and demographic characteristicsa
| Characteristic | Value(s) for participants receiving: |
||
|---|---|---|---|
| Ritonavir | Ketoconazole | EE-LNG | |
| No. (%) of participants enrolled | 8 | 10 | 20 |
| Mean (range) age (yr) | 29.4 (21–46) | 34 (22–50) | 54 (42–65) |
| No. (%) of subjects by sex | |||
| Male | 8 (100) | 8 (80) | 0 |
| Female | 0 | 2 (20) | 20 (100) |
| No. (%) of participants by race | |||
| White | 8 (100) | 7 (70) | 18 (90) |
| Black or African-American | 0 | 3 (30) | 2 (10) |
| Mean (range) wt (kg) | 71.6 (57.0–85.0) | 78.6 (73.5–92.8) | 69.7 (53.3–81.3) |
| Mean (range) ht (cm) | 180.1 (174–187) | 175.7 (163.0–187.0) | 160.7 (153.0–175.0) |
| Mean (range) BMI (kg/m2) | 22.1 (17.8–25.1) | 25.6 (21.1–30.3) | 27.1 (21.0–31.1) |
| No. of participants who: | |||
| Completed the trial | 8 | 10 | 19 |
| Discontinued the trial | 0 | 0 | 1 |
None of the participants were infected with HIV. BMI, body mass index; HIV, human immunodeficiency virus.
TABLE 3.
EE and LNG PK results: effect of doravirine on PK of CYP3A substratesa
| Oral contraceptive ± trial drugs | Values for the following PK parameter: |
|||
|---|---|---|---|---|
| AUC0–∞b | Cmaxb | Tmax (h)c | t1/2 (h)d | |
| EE | ||||
| EE-LNG (n = 20)e | 845.66 (758.43, 942.92) | 66.03 (59.09, 73.78) | 1.50 (1.00, 2.00) | 18.6 (17.5) |
| EE-LNG + doravirine (n = 19)e | 832.32 (750.20, 923.43) | 55.12 (49.30, 61.63) | 1.50 (1.00, 3.00) | 18.8 (16.6) |
| (EE-LNG + doravirine)/EE-LNGf | 0.98 (0.94, 1.03) | 0.83 (0.80, 0.87) | ||
| LNG | ||||
| EE-LNG (n = 20)e | 37.72 (30.77, 46.23) | 2.57 (2.13, 3.09) | 1.27 (1.01, 4.05) | 37.7 (31.2) |
| EE-LNG + doravirine (n = 19)e | 45.59 (36.39, 57.12) | 2.47 (2.02, 3.01) | 1.51 (0.51, 6.00) | 43.0 (34.3) |
| (EE-LNG + doravirine)/EE-LNGf | 1.21 (1.14, 1.28) | 0.96 (0.88, 1.05) | ||
AUC0–∞, area under the concentration-time curve from time zero extrapolated to infinity; Cmax, maximum plasma concentration; EE, ethinyl estradiol; LNG, levonorgestrel; PK, pharmacokinetic; t1/2, apparent terminal half-life; Tmax, time to reach Cmax.
Back-transformed least-squares mean and confidence intervals are from a linear mixed-effects model performed on l-transformed values. Data for AUC0–∞ are in picograms·hour per milliliter for EE and nanograms·hour per milliliter for LNG, and data for Cmax are in picograms per milliliter for EE and nanograms per milliliter for LNG.
The median (minimum, maximum) is reported for Tmax.
The geometric mean (percent geometric coefficient of variation) is reported for t1/2. The geometric coefficient of variation was calculated in the ln scale with the equation , where s2 is the observed variance on the natural log scale.
Data represent the geometric least-squares mean (95% confidence interval) unless indicated otherwise.
Data represent the geometric least-squares mean ratio (90% confidence interval) unless indicated otherwise.
Effect of multiple-dose ritonavir on doravirine PK.
Eight healthy men were enrolled and completed the trial (Table 1). The baseline demographic characteristics are shown in Table 2, and the summary statistics for doravirine PK are shown in Table 4. The doravirine AUC0–∞ and plasma concentration at 24 h postdose (C24) increased approximately 3-fold following coadministration of ritonavir compared with the values obtained following the administration of doravirine alone, while the maximum plasma concentration (Cmax) increased by only ∼30% during coadministration. The increases in doravirine AUC0–∞ and C24 are consistent with the increase in the geometric mean (GM) apparent terminal half-life (t1/2) from approximately 14 h after administration of doravirine alone to 35 h with doravirine coadministered with ritonavir. The coadministration of doravirine with multiple doses of ritonavir was generally well tolerated. Eight participants (100%) reported 22 AEs, all of which were mild or moderate in intensity and had resolved by the end of the trial, with the exception of two unrelated AEs, lipoma and urticaria, which had not resolved by the end of the trial. The most common AE was headache, which was reported by three participants (37.5%); no other AE was reported by more than one participant. Five participants (62%) reported 11 AEs that were deemed to be drug related: four participants reported treatment-related AEs following treatment with ritonavir, and two participants reported treatment-related AEs following treatment with ritonavir-doravirine. Of these drug-related AEs, the most common was headache (two participants, 25%); no other drug-related AE was reported by more than one participant. There were no SAEs, deaths, or discontinuations due to AEs and no clinically meaningful changes in laboratory tests, vital signs, or electrocardiograms.
TABLE 4.
Doravirine PK results: effect of CYP3A inhibitors on doravirine PKa
| Trial drug | PK parameter |
||||
|---|---|---|---|---|---|
| AUC0–∞ (μM·h)b | Cmax (nM)b | C24 (nM)b | Tmax (h)c | t1/2 (h)d | |
| Ritonavir | |||||
| Doravirine at 50 mg (n = 8)e | 20.8 (17.5, 24.6) | 963 (825, 1,120) | 322 (266, 390) | 3.50 (2.00, 5.00) | 14.0 (10.6) |
| Doravirine + ritonavir (n = 8)e | 73.5 (62.0, 87.1) | 1,260 (1,080, 1,470) | 935 (772, 1,130) | 5.00 (1.00, 16.00) | 35.2 (12.3) |
| (Doravirine + ritonavir)/doravirinef | 3.54 (3.04, 4.11) | 1.31 (1.17, 1.46) | 2.91 (2.33, 3.62) | ||
| Ketoconazole | |||||
| Doravirine at 100 mg (n = 10)e | 29.88 (26.61, 33.56) | 1,402.12 (1,160.00, 1694.77) | 429.51 (382.57, 482.21) | 2.00 (1.00, 6.00) | 15.2 (28.1) |
| Doravirine + ketoconazole (n = 10)e | 91.47 (76.36, 109.56) | 1,759.00 (1,460.93, 2,117.89) | 1,180.14 (991.41, 1,404.80) | 3.00 (1.00, 24.00) | 32.4 (12.5) |
| (Doravirine + ketoconazole)/doravirinef | 3.06 (2.85, 3.29) | 1.25 (1.05, 1.49) | 2.75 (2.54, 2.98) | ||
AUC0–∞, area under the plasma concentration-time curve from time zero extrapolated to infinity; C24, plasma concentration at 24 h postdose; Cmax, maximum plasma concentration; CYP3A, cytochrome P450 3A; PK, pharmacokinetic; t1/2, apparent terminal half-life; Tmax, time to Cmax.
Back-transformed least-squares mean and confidence intervals are from a linear mixed-effects model performed on natural log-transformed values.
The median (minimum, maximum) is reported for Tmax.
The geometric mean (percent geometric coefficient of variation) is reported for t1/2.
Data represent the geometric least-squares mean (95% confidence interval) unless indicated otherwise.
Data represent the geometric least-squares mean ratio (90% confidence interval) unless indicated otherwise.
Effect of multiple-dose ketoconazole on doravirine PK.
Ten participants (eight men, two women) were enrolled and completed the trial (Table 1). The baseline demographic characteristics are shown in Table 2, and the summary statistics for doravirine plasma PK are shown in Table 4. The coadministration of doravirine and ketoconazole led to an ∼3-fold increase in the doravirine AUC0–∞ and C24 and an ∼25% increase in the doravirine Cmax compared with values achieved after administration of doravirine alone. The GM apparent terminal t1/2 was greater following doravirine and ketoconazole coadministration (∼32 h) than following the administration of doravirine alone (∼15 h). Doravirine was generally well tolerated both alone and with multiple doses of ketoconazole. Six of 10 participants (60%) reported a total of 18 mild AEs, all of which were resolved by the end of the trial. The most common AEs were nausea (two participants, 20%), rhinorrhea (two participants, 20%), and sinus congestion (two participants, 20%). Thirteen events were considered drug related: six were related to doravirine only, five were related to ketoconazole only, and two were related to both drugs. Of these drug-related AEs, the most common was nausea (two participants, 20%); headache, insomnia, restlessness, rhinorrhea, papule, pruritus, and papular rash were reported by one participant each. Only mild AEs were reported, with no occurrence of SAEs, deaths, discontinuations, or laboratory AEs. No clinically meaningful changes in laboratory tests, vital signs, or electrocardiograms were observed.
DISCUSSION
Doravirine is an NNRTI indicated for the once-daily treatment of HIV-1 infection in combination with other ARTs (11, 12). Due to the prevalence of comorbidities with HIV-1 infection, doravirine is anticipated to be coadministered with a range of concomitant treatments. In vitro data have indicated that the main metabolizing enzyme for doravirine is CYP3A (16). Doravirine metabolism was not expected to be affected by the modulation of any other major CYPs or transporters; however, strong inhibitors and inducers of CYP3A may have a relevant effect on the PK of doravirine. Therefore, the potential for doravirine to be a victim or perpetrator of CYP3A-mediated DDIs was evaluated in dedicated clinical trials.
Effect of doravirine on the PK of CYP3A substrates.
Preclinical data indicate that doravirine is not an inhibitor of major CYP enzymes and has minimal potential to induce CYP3A activity (17). Additionally, autoinduction was not observed in a trial comparing a single dose and 10 days of multiple dosing of doravirine (10). Furthermore, area under the concentration-time curve from time zero to 24 h postdose (AUC0–24) accumulation ratios of 1.2 to 1.4 after daily dosing are consistent with the apparent terminal t1/2 and indicate no evidence of time-dependent PK, such as autoinduction or autoinhibition (10). Preclinical data also support the suggestion that doravirine is not a clinically relevant inhibitor of major transporters, including P-gp, OATP1B1/3, OCT2, and OAT1/3 (17). In vitro data suggest that doravirine is not a strong inhibitor of BCRP (17), so systemic exposure to doravirine is unlikely to inhibit the elimination of BCRP substrates. However, based on estimated gut concentrations, there is potential for doravirine to inhibit intestinal BCRP. In a clinical trial at a supratherapeutic dose of 200 mg of doravirine, only an ∼30 to 40% increase in the AUC0–24 and Cmax of the BCRP substrate dolutegravir was observed (40).
Although the likelihood of DDIs was low, the ability of doravirine to be the perpetrator of DDIs through CYP3A interactions was investigated in clinical DDI trials to confirm the preclinical findings. Validating the preclinical data for CYP3A was particularly important, as this enzyme is responsible for the metabolism of many commonly prescribed drugs (41).
Results from the EE-LNG DDI trial indicated that doravirine does not perpetrate DDIs with EE-LNG through effects on CYP3A metabolism or other transporters or metabolic enzymes involved in EE-LNG clearance. Overall, there were no clinically relevant changes in the PK of EE or LNG when EE-LNG was administered with or without doravirine in women of nonchildbearing potential. While the EE AUC0–∞ and Cmax met bioequivalence bounds of [0.8, 1.25] when the oral contraceptive was administered with and without doravirine, as did the Cmax for LNG, the LNG AUC0–∞ was increased slightly (∼21%) following EE-LNG coadministration with doravirine and fell outside the bounds. However, the exposure of LNG was well within the exposures observed for another marketed oral contraceptive product which also contains 0.03 mg EE and 0.15 mg LNG (42); these data support the suggestion that the increased AUC0–∞ value is not clinically relevant. The results indicate that doravirine may be coadministered with oral contraceptives containing EE and LNG without dose adjustment and provide further support that doravirine is not a perpetrator of clinically meaningful DDIs through CYP3A.
Similarly, in a previously reported trial (10), multiple-dose administration of doravirine did not have a clinically relevant effect on the PK of midazolam, a sensitive CYP3A substrate often used as a probe of CYP3A activity and modulation. The ∼18% reduction in midazolam exposure (AUC0–∞ [doravirine + midazolam]/midazolam geometric mean ratio [GMR], 0.82; 90% confidence interval [CI], 0.70, 0.97) observed upon coadministration with multiple doses of doravirine was not considered to be clinically relevant.
The exposure and clearance of the antihypercholesterolemia drug atorvastatin (43), a CYP3A and OATP1B1 substrate that is known to be affected by CYP3A modulation (44), were unaffected by coadministration with doravirine (AUC0–∞ [atorvastatin + doravirine]/atorvastatin GMR, 0.98; 90% CI, 0.90, 1.06), providing further evidence that doravirine does not impact CYP3A-mediated metabolism (21). Although the atorvastatin Cmax was reduced by 33% when it was coadministered with doravirine compared with that when atorvastatin was administered alone (Cmax [atorvastatin + doravirine]/atorvastatin GMR, 0.67; 90% CI, 0.52, 0.85), this difference was not deemed to be clinically meaningful due to efficacy being maintained in other studies with similar decreases in Cmax (21). Previous research showed that reductions in the atorvastatin Cmax of 25% and 31% associated with food intake (45) and nighttime dosing (46), respectively, did not affect its efficacy, while a meta-analysis has indicated that total daily exposure (rather than peak exposure) is correlated with the efficacy of statins (47). The results of the doravirine-atorvastatin trial support the concomitant administration of doravirine and atorvastatin in individuals with HIV-1 infection and hypercholesterolemia, with no evidence of a significant DDI related to CYP3A modulation.
Overall, no clinically relevant effects on the PK of the CYP3A substrates EE-LNG, midazolam, or atorvastatin were observed in the trials described above. Consistent with the findings from in vitro studies (17), these results suggest that doravirine is not a perpetrator of clinically meaningful DDIs via CYP3A.
Effect of CYP3A inhibitors on doravirine PK.
Doravirine exposure was found to be increased by the strong CYP3A inhibitors ritonavir and ketoconazole, with the increases in AUC0–∞ being approximately 3-fold (Table 4). Though the ritonavir DDI trial was conducted with a low dose of doravirine (50 mg, compared with the clinical dose of 100 mg), the almost linear PK of doravirine (10) allow for the extrapolation of the results to a 100-mg dose of doravirine. The observed increase in doravirine exposure in these trials is consistent with data obtained in the AME trial, which indicated oxidative metabolism as the major route of elimination of doravirine, and with preclinical data suggesting that CYP3A is the main enzyme responsible for doravirine metabolism (16). However, the size of the effect was modest compared with the effect of the sensitive CYP3A substrate midazolam (which has shown increases in exposure of ∼28-fold when coadministered with ritonavir [48]), likely due to the low intrinsic clearance of doravirine, which is reflected by a low systematic clearance and a minimal first-pass effect (16). Ritonavir and ketoconazole are also a potential mixed inducer/inhibitor and a strong inhibitor of P-gp, respectively (30, 38), and could potentially affect doravirine PK via this transporter. The coadministration of ritonavir or ketoconazole caused an increase in the doravirine Cmax of only 31% and 25%, respectively. The limited increases in the doravirine Cmax, despite it being a P-gp substrate, were anticipated based on the high apparent permeability (25 × 10−6 cm/s [16]) of doravirine observed in vitro added to the low first-pass effect. In addition, doravirine was not a substrate of OATP1B1/3 in vitro (17), meaning that it was unlikely to be affected by inhibition of OATP1B1/3 by ritonavir and ketoconazole (49, 50). These data further support the suggestion that the increased doravirine exposure in the presence of ritonavir and ketoconazole is likely due to decreased clearance via CYP3A inhibition, rather than through inhibition of P-gp or other drug transporters.
While coadministration with strong CYP3A inhibitors may increase doravirine exposure, the clinical experience with doravirine across phase 1, 2, and 3 clinical development suggests that the increases are not likely to be clinically meaningful (10, 51, 52). Safety and tolerability data are available from short-term, high-dose phase 1 trials of doravirine, where singles doses of up to 1,200 mg and multiple doses of up to 750 mg once daily for 10 days were administered. In these trials, up to ∼6.4-fold increases in exposure over the projected therapeutic exposure were achieved, with good tolerability and no safety issues being identified (10). Additionally, a thorough QT trial using a dose of 1,200 mg, corresponding with an ∼3.5-fold exposure and an ∼4-fold Cmax, showed no meaningful effect of a supratherapeutic dose of doravirine on the heart rate-corrected QT interval (51). Moreover, safety and tolerability data from the 200-mg dose in the 24-week phase 2 dose-ranging trial (which provided an exposure nearly 2-fold higher than the projected therapeutic dose) (52) did not reveal any safety issues of concern. Overall, the clinical experience at higher exposures of doravirine relative to that with the anticipated clinical dose indicates that these increased exposures are well tolerated. As a result, these data support the permitted use of strong CYP3A inhibitors in phase 3 trials with doravirine (13, 14). As the increases in doravirine exposure seen with strong CYP3A inhibitors represent the largest potential effect on doravirine exposure by CYP3A inhibitors, these data also support the coadministration of doravirine with moderate inhibitors of CYP3A.
Effect of CYP3A inducers on doravirine PK.
Due to the role of CYP3A in doravirine metabolism, CYP3A inducers can reduce doravirine exposure. The effects of the CYP3A inducers rifampin, rifabutin, and efavirenz on the PK of doravirine were examined in three previously published trials (18–20), and decreased exposure to doravirine was observed when it was coadministered with rifampin, rifabutin, or efavirenz.
Rifampin is used to treat tuberculosis, the leading cause of death among people living with HIV-1 infection (7, 53). Rifampin is an acute inhibitor of intestinal P-gp and hepatic OATP1B1/1B3 after single-dose administration (54) and is a potent inducer of CYP3A and intestinal P-gp after multiple-dose administration (54). Following coadministration of multiple doses of rifampin (600 mg once daily for 15 days) with doravirine (a 100-mg single dose on day 14), the plasma AUC0–∞, Cmax, and C24 of doravirine were significantly decreased by 88%, 57%, and 97%, respectively, via induction of CYP3A (AUC0–∞, Cmax, and C24 [doravirine + rifampin]/doravirine GMR, 0.12 [90% CI, 0.10, 0.15], 0.43 [90% CI, 0.35, 0.52], and 0.03 [90% CI, 0.02, 0.04], respectively). This finding is consistent with CYP3A being the primary pathway responsible for doravirine clearance (16). Based on the significant reduction in doravirine PK, coadministration of multiple doses of rifampin will likely reduce the efficacy of doravirine to below the in vitro and in vivo efficacy targets, and, hence, coadministration of these two agents cannot be supported (18).
These results were in contrast to those achieved by the coadministration of a single dose of rifampin with doravirine (which was intended to probe the effect of P-gp and OATP1B1/3 inhibition on doravirine PK), which had little impact on doravirine PK (18). The doravirine AUC0–∞ and C24 were largely unaffected, while the doravirine Cmax was increased by approximately 40% when single-dose rifampin was coadministered with doravirine compared with that when doravirine was administered alone. Similar to the increase in Cmax observed with ritonavir and ketoconazole, this increase in Cmax with single-dose rifampin is likely due to the small impact of P-gp inhibition, which is mitigated by the high permeation of doravirine. OATP1B1 inhibition by rifampin was thought to be unlikely to cause an interaction due to doravirine not being an OATP1B1 substrate (17).
Rifabutin is a more modest inducer of CYP3A than rifampin (55, 56) and also has a lesser inducing effect on P-gp gene expression than rifampin (55). The coadministration of multiple-dose rifabutin (300 mg once daily for 16 days) and doravirine (a 100-mg single dose on day 14) reduced the exposure and C24 of doravirine—albeit to a lesser extent than the reductions observed with rifampin—by 50% and 68%, respectively, though Cmax remained unchanged (AUC0–∞, Cmax, and C24 [doravirine + rifabutin]/doravirine GMR for doravirine, 0.50 [90% CI, 0.45, 0.55], 0.99 [90% CI, 0.85, 1.15], and 0.32 [90% CI, 0.28, 0.35], respectively) (19). However, it has been demonstrated previously (19) via nonparametric superposition that increasing the frequency of doravirine dosing from 100 mg once daily to 100 mg twice daily results in C24 values that are comparable to those achieved with a dose of doravirine at 100 mg once daily without coadministration of rifabutin. These findings suggest that decreases in doravirine exposure when it is coadministered with rifabutin can be addressed by dose adjustment.
Efavirenz, an NNRTI used for the treatment of HIV-1 infection in certain clinical situations (9), is an inducer of hepatic CYP3A, though it is a less potent inducer than rifampin (57). It is known that the use of efavirenz is associated with AEs, including those of the central nervous system (9, 58). For individuals who do not tolerate efavirenz, a doravirine-based regimen may be suitable. However, the moderate CYP3A-inducing effect of efavirenz is expected to persist for several days following the cessation of therapy (59). Following a switch from efavirenz (600 mg once daily) to doravirine (100 mg once daily) in a PK study of healthy adults (20), doravirine exposure on day 1 following efavirenz cessation was reduced substantially by 62% compared with that achieved with doravirine administered without preceding efavirenz treatment, as was expected due to the CYP3A-inducing nature of efavirenz (day 1 doravirine AUC0–24 with prior efavirenz/doravirine without prior efavirenz treatment GMR, 0.38; 90% CI, 0.33, 0.45). However, as efavirenz was washed out, its inducing effect on doravirine PK diminished over the 14 days following the cessation of efavirenz, during which doravirine concentrations were measured (day 14 doravirine AUC0–24 with prior efavirenz/doravirine without prior efavirenz treatment GMR, 0.68; 90% CI, 0.58, 0.80). Doravirine C24 values exceeding the in vitro efficacy target of 78 nM were reached at day 2 following efavirenz cessation. However, efavirenz was maintained at therapeutic concentrations of >1,000 ng/ml until day 4 after efavirenz cessation (the reported therapeutic range for efavirenz is 1,000 to 4,000 ng/ml [60]). Therefore, dose adjustment may not be necessary following a switch from efavirenz to doravirine, as the therapeutic concentrations of at least one of the NNRTIs appeared to be maintained during the transition period. This proposal assumes that the target for doravirine efficacy of at least 6-fold above the in vitro 50% effective concentration (61) translates to efficacy. People living with HIV are also expected to be virally suppressed and continue to receive two additional ART drugs as part of their therapy during a switch in therapy (9), which further supports the low risk for resistance or viral breakthrough when the concentration of a single ART drug (e.g., doravirine) may be below target clinical concentrations for a brief period. The clinical relevance of this transient interaction has been further investigated in a phase 3 trial (MK-1439A protocol 024, ClinicalTrials.gov registration number NCT02397096) in virally suppressed participants switching from a continuous antiretroviral regimen to a single-tablet regimen of doravirine at 100 mg with lamivudine at 300 mg and tenofovir disoproxil fumarate at 300 mg for 48 weeks (62).
Based on these data from clinical trials with strong and moderate inducers, doravirine should not be coadministered with strong inducers (for example, the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, and phenytoin; the antimycobacterials rifampin and rifapentine; St. John’s wort [Hypericum perforatum]). However, for the case of rifabutin described above, dose adjustment of doravirine may be an acceptable means to counteract the reduction in doravirine exposure resulting from the concomitant use of CYP3A inducers. As the magnitude of induction associated with CYP3A moderate inducers may vary between compounds, as shown here by rifabutin and efavirenz, and is also dependent on the substrate, the effect of other CYP3A inducers on the PK of doravirine cannot easily be predicted. Therefore, if coadministration with moderate inducers cannot be avoided, the frequency of doravirine dosing should be increased from 100 mg once daily to 100 mg twice daily. Examples of moderate CYP3A inducers which may require dose adjustment include nafcillin (63), bosentan (64), dabrafenib (65), and lesinurad (66).
Limitations.
All of the studies discussed were performed in healthy adults under controlled conditions. However, the PK of doravirine are similar irrespective of HIV-1 infection status (67), fed or fasted state (68), gender (69), and age (69). Therefore, these results are expected to be clinically representative.
Conclusions.
The results from DDI trials with CYP3A substrates in healthy adults support the use of doravirine with medications metabolized by CYP3A, as clinically relevant changes in the PK of these concomitant medications are not expected. Overall, while doravirine PK are affected by CYP3A inhibitors and inducers, the concomitant use of doravirine with CYP3A inhibitors is not of concern. Doravirine should not be coadministered with strong inducers but may be used with dose regimen adjustment with rifabutin; however, the effect of other moderate inducers on doravirine PK is unknown.
MATERIALS AND METHODS
Trial design.
An overview of the trials studying the coadministration of doravirine and the CYP3A modulators ritonavir and ketoconazole or the CYP3A substrates EE and LNG can be found in Table 1.
The trials were conducted in accordance with principles of good clinical practice and were approved by the appropriate institutional review boards and regulatory agencies (for the EE-LNG and ketoconazole trials, Chesapeake Research Review, Inc., Columbia, MD, USA; for the ritonavir trial, the Ethics Committee of the University Hospital Ghent [UZGent], Ghent, Belgium). Written informed consent was obtained from all participants.
Sample collection and assay conditions.
Blood samples for assay of doravirine plasma concentration were collected at the time points given in Table 1. Samples were centrifuged at 4°C at 1,000 to 3,000 relative centrifugal force for 10 min to isolate the plasma, which was then stored at –20°C until analysis.
(i) EE-LNG trial.
EE and LNG plasma concentrations were determined via validated liquid chromatography-mass spectrometry methods (Pharmanet Canada Inc., QC, Canada). The lower limits of quantification (LLOQs) were 1.00 pg/ml for EE (analytical range, 1.00 to 200.40 pg/ml) and 25.00 pg/ml for LNG (analytical range, 25.00 to 5,000.00 pg/ml).
(ii) Ritonavir and ketoconazole trials.
Doravirine plasma concentrations were determined via validated reverse-phase liquid chromatography with tandem mass spectrometry (Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA). The LLOQ was 1.0 ng/ml (analytical range, 1.00 to 1,000 ng/ml).
PK parameters.
For the EE-LNG trial, the values of the PK parameters, including AUC0–∞, Cmax, the time to reach Cmax (Tmax), and t1/2, were calculated for EE and LNG. In the ritonavir and ketoconazole trials, the values of the doravirine PK parameters, including AUC0–∞, Cmax, C24, Tmax, and t1/2, were calculated.
Doravirine PK parameters for the ketoconazole interaction trial and the EE and LNG PK parameters were calculated using Phoenix WinNonlin Professional (version 5.2) software (Certara USA, Inc., Princeton, NJ, USA). Doravirine PK parameters for the ritonavir interaction trial were calculated using Phoenix WinNonlin (version 6.3) software (Certara USA, Inc., Princeton, NJ, USA). Cmax, Tmax, and C24 (for doravirine) were generated from the plasma concentration-time data. AUC0–∞ was calculated using the linear trapezoidal method for ascending concentrations and the log trapezoidal method for descending concentrations (linear up/log down). λz was calculated by regression of the terminal log-linear portion of the plasma concentration-time profile, and the apparent terminal t1/2 was calculated as the quotient of the natural log of 2 (ln[2]) and λz.
Statistical analysis.
(i) EE-LNG and ketoconazole trials.
Doravirine AUC0–∞, C24, and Cmax values and EE and LNG AUC0–∞ and Cmax values were ln transformed prior to analysis and evaluated separately using a linear mixed-effects model with a fixed-effect term for treatment. An unstructured covariance matrix was used to allow for unequal treatment variances and model the correlation between the two treatment measurements within each participant. Tmax and apparent terminal t1/2 were summarized using descriptive statistics.
In the EE-LNG trial, the one participant (5%) who discontinued prior to dosing on day 4 of period 2 due to an AE (elevated blood pressure) was not included in the PK and statistical analysis for period 2 (EE-LNG and doravirine coadministration) due to incomplete data and was included only in the safety analysis population.
(ii) Ritonavir trial.
Individual values of the doravirine AUC0–∞, C24, and Cmax were ln transformed and analyzed by using a linear mixed-effects model containing treatment as a fixed effect and subject as a random effect. The two-sided 90% CI for the GMR ([doravirine + ritonavir]/doravirine) of the doravirine AUC0–∞, C24, and Cmax was generated from the model described above. The 90% CI for the GMR of doravirine C24 was compared against the prespecified bound of (0.50, 2.00).
Safety.
Safety was monitored throughout the trials via clinical and laboratory evaluations and AE monitoring.
Data availability.
The data-sharing policy of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.
ACKNOWLEDGMENTS
We thank the trial participants, clinical trial site staff, Ernestina Aboagye, Danielle Armas, Chris Chung, Ying Guo, Rachael Liu, and David Kaufman for their contribution to this work.
Medical writing assistance, under the direction of the authors, was provided by Kirsty Muirhead of CMC Affinity, a division of McCann Health Medical Communications Ltd, Glasgow, UK, in accordance with good publication practice (GPP3) guidelines. This assistance was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Funding for this research was provided by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
S.G.K., K.L.Y., R.I.S., L.F., M.S.A., M.S., T.L., and M.I. are current or former employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA. L.V.B. and G.V.L. are part of the Drug Research Unit Ghent (D.R.U.G.), a department of University Hospital Ghent. D.R.U.G. has been paid for conducting early-phase clinical trials with doravirine (contract research). S.R. has no disclosures to declare.
M.S.A., L.F., S.G.K., T.L., M.S., R.I.S., and K.L.Y. conceived of or designed the work. S.R., T.L., M.S., L.V.B., and G.V.L. acquired data. M.S.A., L.F., and K.L.Y. performed the data analysis. M.S.A., L.F., M.I., S.G.K., K.L.Y., and G.V.L. interpreted the data. All authors contributed to the writing and/or reviewing of the manuscript and approved the final version.
REFERENCES
- 1.Kaulich-Bartz J, Dam W, May MT, Lederberger B, Widmer U, Phillips AN, Grabar S, Mocroft A, Vilaro J, van Sighem A, Moreno S, Dabis F, Monforte AD, Teira R, Ingle SM, Sterne JA. 2013. Insurability of HIV-positive people treated with antiretroviral therapy in Europe: collaborative analysis of HIV cohort studies. AIDS 27:1641–1655. doi: 10.1097/QAD.0b013e3283601199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trickey A, May MT, Vehreschild JJ, Obel N, Gill MJ, Crane HM, Boesecke C, Patterson S, Grabar S, Cazanave C, Cavassini M, Shepherd L, Monforte AD, van Sighem A, Saag M, Lampe F, Hernando V, Montero M, Zangerle R, Justice AC, Sterling T, Ingle SM, Sterne JAC, Boulle A, Stephan C, Miro JM, Chêne G, Costagliola D, Dabis F, del Amo J, Guest J, Haerry DH-U, Hogg R, Justice A, Smith C, Reiss P, Saag M, Teira R, Williams M, May MT. 2017. Survival of HIV-positive patients starting antiretroviral therapy between 1996 and 2013: a collaborative analysis of cohort studies. Lancet HIV 4:e349–e356. doi: 10.1016/S2352-3018(17)30066-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hasse B, Ledergerber B, Furrer H, Battegay M, Hirschel B, Cavassini M, Bertisch B, Bernasconi E, Weber R. 2011. Morbidity and aging in HIV-infected persons: the Swiss HIV cohort study. Clin Infect Dis 53:1130–1139. doi: 10.1093/cid/cir626. [DOI] [PubMed] [Google Scholar]
- 4.Vance DE, Mugavero M, Willig J, Raper JL, Saag MS. 2011. Aging with HIV: a cross-sectional study of comorbidity prevalence and clinical characteristics across decades of life. J Assoc Nurses AIDS Care 22:17–25. doi: 10.1016/j.jana.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Tseng A, Szadkowski L, Walmsley S, Salit I, Raboud J. 2013. Association of age with polypharmacy and risk of drug interactions with antiretroviral medications in HIV-positive patients. Ann Pharmacother 47:1429–1439. doi: 10.1177/1060028013504075. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell HS, Stephens E. 2004. Contraception choice for HIV positive women. Sex Transm Infect 80:167–173. doi: 10.1136/sti.2003.008441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu E, Makubi A, Drain P, Spiegelman D, Sando D, Li N, Chalamilla G, Sudfeld CR, Hertzmark E, Fawzi WW. 2015. Tuberculosis incidence rate and risk factors among HIV-infected adults with access to antiretroviral therapy. AIDS 29:1391–1399. doi: 10.1097/QAD.0000000000000705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC). 2017. HIV and viral hepatitis. https://www.cdc.gov/hiv/pdf/library/factsheets/hiv-viral-hepatitis.pdf.
- 9.Panel on Antiretroviral Guidelines for Adults and Adolescents. 2018. Guidelines for the use of antiretroviral agents in adults and adolescents living with HIV. U.S. Department of Health and Human Services, Washington, DC: https://aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 10.Anderson MS, Gilmartin J, Cilissen C, De Lepeleire I, van Bortel L, Dockendorf MF, Tetteh E, Ancona JK, Liu R, Guo Y, Wagner JA, Butterton JR. 2015. Safety, tolerability and pharmacokinetics of doravirine, a novel HIV non-nucleoside reverse transcriptase inhibitor, after single and multiple doses in healthy subjects. Antivir Ther 20:397–405. doi: 10.3851/IMP2920. [DOI] [PubMed] [Google Scholar]
- 11.Merck Sharp & Dohme Corp. 2018. Pifeltro (doravirine) prescribing information. Merck & Co., Inc, Whitehouse Station, NJ: https://www.merck.com/product/usa/pi_circulars/p/pifeltro/pifeltro_pi.pdf. [Google Scholar]
- 12.Merck Sharp & Dohme Corp. 2018. Delstrigo (doravirine, lamivudine, and tenofovir disoproxil fumarate) prescribing information. Merck & Co., Inc., Whitehouse Station, NJ: https://www.merck.com/product/usa/pi_circulars/d/delstrigo/delstrigo_pi.pdf. [Google Scholar]
- 13.Molina JM, Squires K, Sax PE, Cahn P, Lombaard J, DeJesus E, Lai MT, Xu X, Rodgers A, Lupinacci L, Kumar S, Sklar P, Nguyen BY, Hanna GJ, Hwang C, DRIVE-FORWARD Study Group. 2018. Doravirine versus ritonavir-boosted darunavir in antiretroviral-naive adults with HIV-1 (DRIVE-FORWARD): 48-week results of a randomised, double-blind, phase 3, non-inferiority trial. Lancet HIV 5:e211–e220. doi: 10.1016/S2352-3018(18)30021-3. [DOI] [PubMed] [Google Scholar]
- 14.Orkin C, Squires KE, Molina JM, Sax PE, Wong WW, Sussmann O, Kaplan R, Lupinacci L, Rodgers A, Xu X, Lin G, Kumar S, Sklar P, Nguyen BY, Hanna GJ, Hwang C, Martin EA, DRIVE-AHEAD Study Group. 2019. Doravirine/lamivudine/tenofovir disoproxil fumarate is non-inferior to efavirenz/emtricitabine/tenofovir disoproxil fumarate in treatment-naive adults with human immunodeficiency virus-1 infection: week 48 results of the DRIVE-AHEAD trial. Clin Infect Dis 68:535–544. doi: 10.1093/cid/ciy540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng M, Sachs NA, Xu M, Grobler J, Blair W, Hazuda DJ, Miller MD, Lai M-T. 2016. Doravirine suppresses common nonnucleoside reverse transcriptase inhibitor-associated mutants at clinically relevant concentrations. Antimicrob Agents Chemother 60:2241–2247. doi: 10.1128/AAC.02650-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez RI, Fillgrove KL, Yee KL, Liang Y, Lu B, Tatavarti A, Liu R, Anderson MS, Behm MO, Fan L, Li Y, Butterton JR, Iwamoto M, Khalilieh SG. 2019. Characterisation of the absorption, distribution, metabolism, excretion and mass balance of doravirine, a non-nucleoside reverse transcriptase inhibitor in humans. Xenobiotica 49:422–432. doi: 10.1080/00498254.2018.1451667. [DOI] [PubMed] [Google Scholar]
- 17.Bleasby K, Fillgrove KL, Houle R, Lu B, Palamanda J, Newton DJ, Lin M, Chan GH, Sanchez RI. 2019. In vitro evaluation of the drug interaction potential of doravirine. Antimicrob Agents Chemother 63:e02492-18. doi: 10.1128/AAC.02492-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yee KL, Khalilieh SG, Sanchez RI, Liu R, Anderson MS, Manthos H, Judge T, Brejda J, Butterton JR. 2017. The effect of single and multiple doses of rifampin on the pharmacokinetics of doravirine in healthy subjects. Clin Drug Investig 37:659–667. doi: 10.1007/s40261-017-0513-4. [DOI] [PubMed] [Google Scholar]
- 19.Khalilieh SG, Yee KL, Sanchez RI, Liu R, Fan L, Martell M, Jordan H, Iwamoto M. 2018. Multiple doses of rifabutin reduce exposure of doravirine in healthy subjects. J Clin Pharmacol 58:1044–1052. doi: 10.1002/jcph.1103. [DOI] [PubMed] [Google Scholar]
- 20.Yee KL, Sanchez RI, Auger P, Liu R, Fan L, Triantafyllou I, Lai MT, Di Spirito M, Iwamoto M, Khalilieh SG. 2017. Evaluation of doravirine pharmacokinetics when switching from efavirenz to doravirine in healthy subjects. Antimicrob Agents Chemother 61:e01757-16. doi: 10.1128/AAC.01757-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khalilieh S, Yee KL, Sanchez RI, Triantafyllou I, Fan L, Maklad N, Jordan H, Martell M, Iwamoto M. 2017. Results of a doravirine-atorvastatin drug-drug interaction study. Antimicrob Agents Chemother 61:e01364-16. doi: 10.1128/AAC.01364-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. 2016. HIV surveillance report—diagnoses of HIV infection in the United States and dependent areas, 2016. https://www.cdc.gov/hiv/pdf/library/reports/surveillance/cdc-hiv-surveillance-report-2016-vol-28.pdf.
- 23.Stanczyk FZ, Roy S. 1990. Metabolism of levonorgestrel, norethindrone, and structurally related contraceptive steroids. Contraception 42:67–96. doi: 10.1016/0010-7824(90)90093-B. [DOI] [PubMed] [Google Scholar]
- 24.Wang B, Sanchez RI, Franklin RB, Evans DC, Huskey SE. 2004. The involvement of CYP3A4 and CYP2C9 in the metabolism of 17 α-ethinylestradiol. Drug Metab Dispos 32:1209–1212. doi: 10.1124/dmd.104.000182. [DOI] [PubMed] [Google Scholar]
- 25.Zhang H, Cui D, Wang B, Han YH, Balimane P, Yang Z, Sinz M, Rodrigues AD. 2007. Pharmacokinetic drug interactions involving 17α-ethinylestradiol: a new look at an old drug. Clin Pharmacokinet 46:133–157. doi: 10.2165/00003088-200746020-00003. [DOI] [PubMed] [Google Scholar]
- 26.Moreno I, Quinones L, Catalan J, Miranda C, Roco A, Sasso J, Tamayo E, Caceres D, Tchernitchin AN, Gaete L, Saavedra I. 2012. Influence of CYP3A4/5 polymorphisms in the pharmacokinetics of levonorgestrel: a pilot study. Biomedica 32:570–577. doi: 10.1590/S0120-41572012000400012. [DOI] [PubMed] [Google Scholar]
- 27.Kirby BJ, Collier AC, Kharasch ED, Whittington D, Thummel KE, Unadkat JD. 2011. Complex drug interactions of HIV protease inhibitors 1: inactivation, induction, and inhibition of cytochrome P450 3A by ritonavir or nelfinavir. Drug Metab Dispos 39:1070–1078. doi: 10.1124/dmd.110.037523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sevrioukova IF, Poulos TL. 2010. Structure and mechanism of the complex between cytochrome P4503A4 and ritonavir. Proc Natl Acad Sci U S A 107:18422–18427. doi: 10.1073/pnas.1010693107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Youle M. 2007. Overview of boosted protease inhibitors in treatment-experienced HIV-infected patients. J Antimicrob Chemother 60:1195–1205. doi: 10.1093/jac/dkm364. [DOI] [PubMed] [Google Scholar]
- 30.Kumar P, Gordon LA, Brooks KM, George JM, Kellogg A, McManus M, Alfaro RM, Nghiem K, Lozier J, Hadigan C, Penzak SR. 2017. Differential influence of the antiretroviral pharmacokinetic enhancers ritonavir and cobicistat on intestinal P-glycoprotein transport and the pharmacokinetic/pharmacodynamic disposition of dabigatran. Antimicrob Agents Chemother 61:e01201-17. doi: 10.1128/AAC.01201-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hsu A, Granneman GR, Bertz RJ. 1998. Ritonavir. Clinical pharmacokinetics and interactions with other anti-HIV agents. Clin Pharmacokinet 35:275–291. doi: 10.2165/00003088-199835040-00002. [DOI] [PubMed] [Google Scholar]
- 32.Zhang D, Chando TJ, Everett DW, Patten CJ, Dehal SS, Humphreys WG. 2005. In vitro inhibition of UDP glucuronosyltransferases by atazanavir and other HIV protease inhibitors and the relationship of this property to in vivo bilirubin glucuronidation. Drug Metab Dispos 33:1729–1739. doi: 10.1124/dmd.105.005447. [DOI] [PubMed] [Google Scholar]
- 33.Yong WP, Ramirez J, Innocenti F, Ratain MJ. 2005. Effects of ketoconazole on glucuronidation by UDP-glucuronosyltransferase enzymes. Clin Cancer Res 11:6699–6704. doi: 10.1158/1078-0432.CCR-05-0703. [DOI] [PubMed] [Google Scholar]
- 34.Daneshmend TK, Warnock DW. 1988. Clinical pharmacokinetics of ketoconazole. Clin Pharmacokinet 14:13–34. doi: 10.2165/00003088-198814010-00002. [DOI] [PubMed] [Google Scholar]
- 35.Outeiro N, Hohmann N, Mikus G. 2016. No increased risk of ketoconazole toxicity in drug-drug interaction studies. J Clin Pharmacol 56:1203–1211. doi: 10.1002/jcph.795. [DOI] [PubMed] [Google Scholar]
- 36.Limper AH, Adenis A, Le T, Harrison TS. 2017. Fungal infections in HIV/AIDS. Lancet Infect Dis 17:e334–e343. doi: 10.1016/S1473-3099(17)30303-1. [DOI] [PubMed] [Google Scholar]
- 37.Food and Drug Administration (FDA). 2013. FDA drug safety communcation: FDA limits usage of Nizoral (ketoconazole) oral tablets due to potentially fatal liver injury and risk of drug interactions and adrenal gland problems. https://www.fda.gov/drugs/drugsafety/ucm362415.htm. [PubMed]
- 38.Wang EJ, Lew K, Casciano CN, Clement RP, Johnson WW. 2002. Interaction of common azole antifungals with P glycoprotein. Antimicrob Agents Chemother 46:160–165. doi: 10.1128/AAC.46.1.160-165.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu Y, She M, Wu Z, Dai R. 2011. The inhibition study of human UDP-glucuronosyltransferases with cytochrome P450 selective substrates and inhibitors. J Enzyme Inhib Med Chem 26:386–393. doi: 10.3109/14756366.2010.518965. [DOI] [PubMed] [Google Scholar]
- 40.Anderson MS, Khalilieh S, Yee KL, Liu R, Fan L, Rizk ML, Shah V, Hussaini A, Song I, Ross LL, Butterton JR. 2017. A two-way steady-state pharmacokinetic interaction study of doravirine (MK-1439) and dolutegravir. Clin Pharmacokinet 56:661–669. doi: 10.1007/s40262-016-0458-4. [DOI] [PubMed] [Google Scholar]
- 41.Zanger UM, Schwab M. 2013. Cytochrome P450 enzymes in drug metabolism: regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol Ther 138:103–141. doi: 10.1016/j.pharmthera.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 42.Duramed Pharmaceuticals Inc. 2010. Seasonique—highlights of prescribing information. https://www.accessdata.fda.gov/drugsatfda_docs/label/2010/021840s008lbl.pdf.
- 43.Husain NE, Ahmed MH. 2015. Managing dyslipidemia in HIV/AIDS patients: challenges and solutions. HIV AIDS (Auckl) 7:1–10. doi: 10.2147/HIV.S46028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Neuvonen PJ. 2010. Drug interactions with HMG-CoA reductase inhibitors (statins): the importance of CYP enzymes, transporters and pharmacogenetics. Curr Opin Investig Drugs 11:323–332. [PubMed] [Google Scholar]
- 45.Whitfield LR, Stern RH, Sedman AJ, Abel R, Gibson DM. 2000. Effect of food on the pharmacodynamics and pharmacokinetics of atorvastatin, an inhibitor of HMG-CoA reductase. Eur J Drug Metab Pharmacokinet 25:97–101. doi: 10.1007/BF03190074. [DOI] [PubMed] [Google Scholar]
- 46.Cilla DD Jr, Gibson DM, Whitfield LR, Sedman AJ. 1996. Pharmacodynamic effects and pharmacokinetics of atorvastatin after administration to normocholesterolemic subjects in the morning and evening. J Clin Pharmacol 36:604–609. doi: 10.1002/j.1552-4604.1996.tb04224.x. [DOI] [PubMed] [Google Scholar]
- 47.Vargo R, Adewale A, Behm MO, Mandema J, Kerbusch T. 2014. Prediction of clinical irrelevance of PK differences in atorvastatin using PK/PD models derived from literature-based meta-analyses. Clin Pharmacol Ther 96:101–109. doi: 10.1038/clpt.2014.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Greenblatt DJ, Peters DE, Oleson LE, Harmatz JS, MacNab MW, Berkowitz N, Zinny MA, Court MH. 2009. Inhibition of oral midazolam clearance by boosting doses of ritonavir, and by 4,4-dimethyl-benziso-(2H)-selenazine (ALT-2074), an experimental catalytic mimic of glutathione oxidase. Br J Clin Pharmacol 68:920–927. doi: 10.1111/j.1365-2125.2009.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annaert P, Ye ZW, Stieger B, Augustijns P. 2010. Interaction of HIV protease inhibitors with OATP1B1, 1B3, and 2B1. Xenobiotica 40:163–176. doi: 10.3109/00498250903509375. [DOI] [PubMed] [Google Scholar]
- 50.Vermeer LM, Isringhausen CD, Ogilvie BW, Buckley DB. 2016. Evaluation of ketoconazole and its alternative clinical CYP3A4/5 inhibitors as inhibitors of drug transporters: the in vitro effects of ketoconazole, ritonavir, clarithromycin, and itraconazole on 13 clinically-relevant drug transporters. Drug Metab Dispos 44:453–459. doi: 10.1124/dmd.115.067744. [DOI] [PubMed] [Google Scholar]
- 51.Khalilieh SG, Yee KL, Fan L, Liu R, Heber W, Dunzo E, Triantafyllou I, Hussaini A, Iwamoto M. 2017. A randomized trial to assess the effect of doravirine on the QTc interval using a single supratherapeutic dose in healthy adult volunteers. Clin Drug Investig 37:975–984. doi: 10.1007/s40261-017-0552-x. [DOI] [PubMed] [Google Scholar]
- 52.Morales-Ramirez JO, Gatell JM, Hagins DP, Thompson M, Arasteh K, Hoffmann C, Harvey C, Xu X, Teppler H. 2014. Safety and antiviral effect of MK-1439, A novel NNRTI (+FTC/TDF) in ART-naive HIV-infected patients, abstr 92LB. Abstr Conf Retroviruses Opportunistic Infections (CROI), Boston, MA. [Google Scholar]
- 53.Wasserman S, Meintjes G. 2014. The diagnosis, management and prevention of HIV-associated tuberculosis. S Afr Med J 104:886–893. doi: 10.7196/SAMJ.9090. [DOI] [PubMed] [Google Scholar]
- 54.Reitman ML, Chu X, Cai X, Yabut J, Venkatasubramanian R, Zajic S, Stone JA, Ding Y, Witter R, Gibson C, Roupe K, Evers R, Wagner JA, Stoch A. 2011. Rifampin's acute inhibitory and chronic inductive drug interactions: experimental and model-based approaches to drug-drug interaction trial design. Clin Pharmacol Ther 89:234–242. doi: 10.1038/clpt.2010.271. [DOI] [PubMed] [Google Scholar]
- 55.Williamson B, Dooley KE, Zhang Y, Back DJ, Owen A. 2013. Induction of influx and efflux transporters and cytochrome P450 3A4 in primary human hepatocytes by rifampin, rifabutin, and rifapentine. Antimicrob Agents Chemother 57:6366–6369. doi: 10.1128/AAC.01124-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Finch CK, Chrisman CR, Baciewicz AM, Self TH. 2002. Rifampin and rifabutin drug interactions: an update. Arch Intern Med 162:985–992. doi: 10.1001/archinte.162.9.985. [DOI] [PubMed] [Google Scholar]
- 57.Mouly S, Lown KS, Kornhauser D, Joseph JL, Fiske WD, Benedek IH, Watkins PB. 2002. Hepatic but not intestinal CYP3A4 displays dose-dependent induction by efavirenz in humans. Clin Pharmacol Ther 72:1–9. doi: 10.1067/mcp.2002.124519. [DOI] [PubMed] [Google Scholar]
- 58.Bristol-Myers Squibb Co. 2017. Sustiva (efavirenz) prescribing information. https://packageinserts.bms.com/pi/pi_sustiva.pdf.
- 59.Crauwels H, Vingerhoets J, Ryan R, Witek J, Anderson D. 2012. Pharmacokinetic parameters of once-daily rilpivirine following administration of efavirenz in healthy subjects. Antivir Ther 17:439–446. doi: 10.3851/IMP1959. [DOI] [PubMed] [Google Scholar]
- 60.Marzolini C, Telenti A, Decosterd LA, Greub G, Biollaz J, Buclin T. 2001. Efavirenz plasma levels can predict treatment failure and central nervous system side effects in HIV-1-infected patients. AIDS 15:71–75. doi: 10.1097/00002030-200101050-00011. [DOI] [PubMed] [Google Scholar]
- 61.Xu Y, Li YF, Zhang D, Dockendorf M, Tetteh E, Rizk ML, Grobler JA, Lai M-T, Gobburu J, Ankrom W. 2016. Characterizing class-specific exposure-viral load suppression response of HIV antiretrovirals using a model-based meta-analysis. Clin Transl Sci 9:192–200. doi: 10.1111/cts.12395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Johnson M, Kumar P, Molina J-M, Rizzardini G, Cahn P, Bickel M, Mallolas J, Zhou Y, Morais C, Kumar S, Sklar P, Hanna G, Hwang C, Greaves W, for the DRIVE-SHIFT Study Group. Switching to doravirine/lamivudine/tenofovir disoproxil fumarate (DOR/3TC/TDF) maintains HIV-1 virologic suppression through 48 weeks: results of the DRIVE-SHIFT trial. J Acquir Immune Defic Syndr, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lang CC, Jamal SK, Mohamed Z, Mustafa MR, Mustafa AM, Lee TC. 2003. Evidence of an interaction between nifedipine and nafcillin in humans. Br J Clin Pharmacol 55:588–590. doi: 10.1046/j.1365-2125.2003.01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dingemanse J, van Giersbergen PL. 2004. Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet 43:1089–1115. doi: 10.2165/00003088-200443150-00003. [DOI] [PubMed] [Google Scholar]
- 65.Puszkiel A, Noe G, Bellesoeur A, Kramkimel N, Paludetto MN, Thomas-Schoemann A, Vidal M, Goldwasser F, Chatelut E, Blanchet B. 9 August 2018. Clinical pharmacokinetics and pharmacodynamics of dabrafenib. Clin Pharmacokinet doi: 10.1007/s40262-018-0703-0. [DOI] [PubMed] [Google Scholar]
- 66.Gillen M, Yang C, Wilson D, Valdez S, Lee C, Kerr B, Shen Z. 2017. Evaluation of pharmacokinetic interactions between lesinurad, a new selective urate reabsorption inhibitor, and CYP enzyme substrates sildenafil, amlodipine, tolbutamide, and repaglinide. Clin Pharmacol Drug Dev 6:363–376. doi: 10.1002/cpdd.324. [DOI] [PubMed] [Google Scholar]
- 67.Schürmann D, Sobotha C, Gilmartin J, Robberechts M, De Lepeleire I, Yee KL, Guo Y, Liu R, Wagner F, Wagner JA, Butterton JR, Anderson MS. 2016. A randomized, double-blind, placebo-controlled, short-term monotherapy study of doravirine in treatment-naive HIV-infected individuals. AIDS 30:57–63. doi: 10.1097/QAD.0000000000000876. [DOI] [PubMed] [Google Scholar]
- 68.Behm MO, Yee KL, Liu R, Levine V, Panebianco D, Fackler P. 2017. The effect of food on doravirine bioavailability: results from two pharmacokinetic studies in healthy subjects. Clin Drug Investig 37:571–579. doi: 10.1007/s40261-017-0512-5. [DOI] [PubMed] [Google Scholar]
- 69.Behm MO, Yee KL, Fan L, Fackler P. 2017. Effect of gender and age on the relative bioavailability of doravirine: results of a phase I trial in healthy subjects. Antivir Ther 22:337–344. doi: 10.3851/IMP3142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data-sharing policy of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, including restrictions, is available at http://engagezone.msd.com/ds_documentation.php. Requests for access to the clinical study data can be submitted through the EngageZone site or via email to dataaccess@merck.com.