Figure 8.
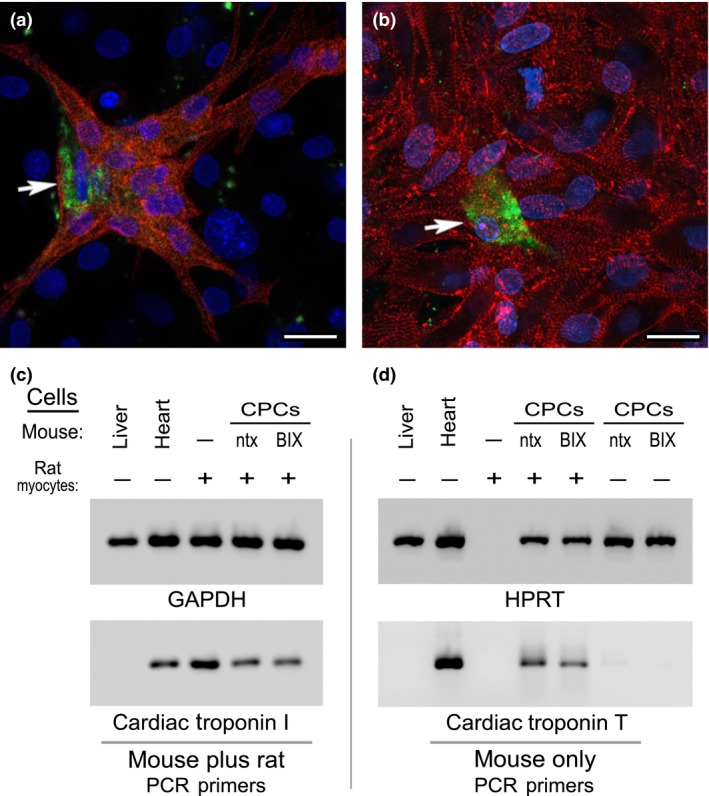
Co‐cultures of mouse CPC s and neonatal rat cardiomyocytes. Phase bright cells collected from (a) non‐treated or (b) BIX01294‐treated atrial tissue were labelled with the fluorescent vital dye CFSE (green), prior to 2 weeks of co‐culture with neonatal rat cardiomyocytes. Afterwards, co‐cultures were immunolabelled against muscle α‐actinin (red) and counterstained with DAPI (blue). Note that under both culture conditions, CPCs were able to undergo cardiac differentiation, as indicated by the presence of CFSE‐labelled, α‐actinin‐positive mouse‐derived cells (arrows) within large clusters of rat myocytes. These two representative images are indicative of the similar cardiac potential of mouse CPCs obtained from non‐treated or BIX01294‐treated atrial explants. Scale bar = 20 μm. This observation was substantiated by measurement of gene expression by PCR amplification of RNA harvested from the co‐cultures. (c) RNA concentrations of mouse liver, mouse heart or rat cardiomyocytes (CM) cultured independently or in the presence of control or BIX01294‐treated mouse CPCs were normalized to the housekeeping gene GAPDH (top panel), using primers that recognize the RNA from both species. Subsequent amplification with non‐species‐specific cardiac troponin I primers (bottom panel) verified that each of the samples (except the negative control liver) displayed strong myocardial gene expression. (d) RNA samples were amplified to the HPRT housekeeping gene using primers that strictly recognize this sequence only in mouse cells (top panel). The concentration of sample loaded per lane was normalized to the expression of HPRT expression, except the rat cardiomyocyte only control. For this rat RNA only sample, template volume was normalized to the co‐culture samples based on the expression displayed in panel C for non‐species‐specific GAPDH. Subsequently, normalized templates were amplified with primers that only recognize cardiac troponin T expressed solely in mouse cells (bottom panel). Among controls, mouse heart displayed expression of this myocardial gene as expected, while neither mouse liver or rat cardiomyocyte template was amplified with these mouse cardiac‐specific primers. However, when co‐cultured with the rat cardiomyocytes, control and BIX01294‐treated mouse CPCs showed near equivalent cardiac troponin T expression. Note that in the absence of the rat cardiomyocytes, neither population of mouse CPCs was positive for cardiac troponin T, which correlates with the progenitor phenotype of the phase bright cell‐derived cells.
