Abstract
Abstract. Objectives: Connexins (Cx) are proteins that form the gap junctional channels at neighbouring plasma membranes between adjacent cells. Cxs are involved in cell communication, which is reportedly correlated with cell proliferation and differentiation. Alterations in connexin expression and/or gap junctional intercellular communication (GJIC) capacity have long been postulated to be important in a number of pathological conditions including cancer. This study was performed to determine the consequences of the deletion of a single allele of Gja1 (Cx43 gene) in Alveolar Type II cells (APTIIs), and its impact on GJIC and cell proliferation. Material and methods: In order to do so, APTIIs from wild type (Cx43+/+) and heterozygous (Cx43+/–) mice were harvested and cultured for 4 days. The GJIC capacity was evaluated by scrape‐loading method, with the transfer of lucifer yellow dye. The expression of Cx43 was evaluated by immunofluorescence method and Western blotting. Cell proliferation was evaluated by 3‐(4,5‐dimethylthazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide assay. Results: It was observed that GJIC capacity was significantly reduced and cell proliferation index was significantly higher in Cx43+/– cells compared to Cx43+/+ cells. Conclusions: These results show that knocking out one allele of Cx43 leads to a lower cell to cell communication capacity, and consequently induces a higher cell proliferation. Because chemically induced lung adenomas in mice are known to originate from APTIIs, these alterations may play a critical role in their susceptibility to lung carcinogenesis.
INTRODUCTION
Gap junctions are clusters of channels formed by special type of proteins named the connexins (Cx) that form the gap junctions. They allow the direct passage of small molecules less than 1 kDa between the cytoplasmic compartments of two adjacent cells (Goodenough et al. 1996). Several molecules can easily diffuse through gap junctions, such as secondary messengers, Ca2+ and IP3 (Saez et al. 1989; Charles et al. 1991; Finkbeiner 1992). About 21 different connexins with different patterns of tissue distribution and developmental expression have been reported.
Gap junctional intercellular communication (GJIC) is known to play an important role in maintaining homeostasis, development and differentiation of many tissues. Impairment of GJIC and decreased Cx expression has been reported to be associated with carcinogenic process (Trosko & Ruch 1998).
Furthermore, studies on reintroduction of connexins expression in cancer cells showed that these proteins have a role in tumour suppression. For example, expression of Cx26 gene has been reported to reduce growth of HeLa cells after transfection (Mesnil et al. 1995). In addition, regenerative growth, tumorigenesis and tumour progression are associated with a reduction in the levels of GJIC (Fehrenbach 2001). Similarly, reduction in gap junction function and expression of Cx genes has been reported using several growth factors, oncogenes and tumour promoters (Ohshima et al. 1985). This reduction or complete loss of GJIC appears to be providing an advantage to cancer cells to escape tissue‐specific growth control signals, and helps in abnormal cellular proliferation. Abnormalities in the expressing GJIC have been reported in several solid tumours namely liver, breast, bladder and skin (Rehm et al. 1988).
There is supporting evidence that vast majority of chemically induced lung tumours and/or spontaneous pulmonary neoplasms originate from alveolar type II epithelial cells (APTIIs) (Hennemann et al. 1992; Traub et al. 1994; Mason et al. 2000). Normally, in lung tissue Cx26, Cx32, Cx43 and Cx46 are expressed (Kasper et al. 1996; Lee et al. 1997; Abraham et al. 1999; Avanzo et al. 2004; King & Lampe 2004), whereas APTII cells in particular express high levels Cx43 (Lee et al. 1997; Abraham et al. 1999). However, their functional role in normal lung as well as during lung tumour development is not clear and still needs to be investigated. Furthermore, recent reports suggests that both Gja1 (Cx43 gene) and Cx32 genes function as tumour suppressors in lung (Avanzo et al. 2004; King & Lampe 2004). Accordingly, in the present study, in order to understand further role of Cx43, we have made an attempt to study the impact of deletion of a single Cx43 allele on GJIC, Cx43 expression patterns and cell proliferation. We wish to report in this study that the deletion of a single Cx43 allele results in the reduction of GJIC capacity in APTII cells, which, is associated with an increase in cell proliferation. Because chemically induced lung adenomas in mice are known to originate from APTIIs, these alterations may play a critical role in their susceptibility to lung carcinogenesis.
MATERIALS AND METHODS
Mice
Cx43+/– mice were obtained from the International Agency for Research on Cancer (IARC, Lyon, France). These mice were generated by replacing the exon‐2 of the Cx43 gene by the neomycin resistance gene (Reaume et al. 1995). They were originally produced in the C57BL/6 strain, but their background was subsequently changed to CD1 by serial breeding at the IARC animal facility. Due to the postnatal lethality of Cx43‐knockout mice, only heterozygous (Cx43+/–) and wild type (Cx43+/+) APTIIs from male mice were used in this study. Cx43expression has been well characterized by real‐time polymerase chain reaction (PCR) and Western blot in these Cx43+/– mice in comparison to Cx43+/+ mice (Avanzo et al. 2004). Heterozygous mice indeed show reduced mRNA amount for Cx43 and lower Cx43 levels.
Genotyping By PCR
DNA from each mouse was obtained from tail biopsies. Genotyping was performed by PCR, as described by Yamakage et al. (1998). The following Cx43 primers were used: sense 5′‐CCCCACTCTCACCTATGTCTCC‐3′ and antisense 5′‐ACTTTTGCCGCCTAGCTATCCC‐3′ observed at 520 bp; neo‐sense 5′‐GGCCACA GTCGATGAATCCAG‐3′ and antisense 5′‐TATCCATCATGGCTGATGCAA‐3′ observed at 294 bp (Fig. 1). After genotyping, the rodents were kept under controlled conditions, from 6 to 10 weeks of age.
Figure 1.
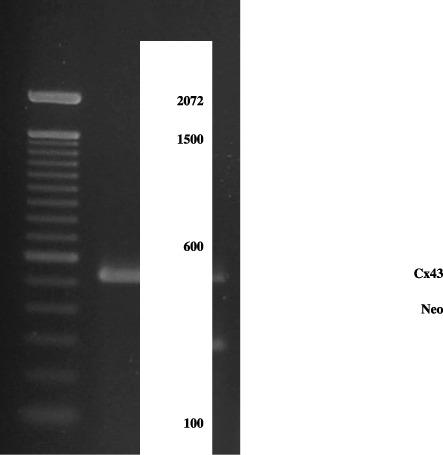
Electrophoresis agarose gel of PCR‐amplified Cx43 and neo‐sequences of DNA extracted of tails from Cx43+/– and Cx43+/+ mice. Bands corresponding to the Cx43 and neo‐sequences are clearly visible after ethydium bromide staining. MW, molecular weight; +/+, Cx43 wild‐type; +/–, Cx43 heterozygous.
Isolation and culture of APTIIS
Raw cell suspensions were prepared from female or male Cx43+/+ and Cx43+/– mouse lungs, using a modification of recently published methods (Corti et al. 1996; Dobbs & Gonzalez 2002; Rice et al. 2002). Briefly, the mice were anaesthetized with Avertin (250–300 µl/10 g of body weight, intraperitoneal injection). The trachea was isolated and cannulated with a 20‐gauge luer stub adapter. The diaphragm was cut open, and the chest plate and thymus were removed. The lungs were perfused with approximately 10 ml of warm saline solution (37 °C) via the right ventricle with a 21‐gauge needle fitted on a 10‐ml syringe. Dispase (DP, 3 ml at 4 °C) (Becton Dickinson Bioscience, Le Pont de Claix, France) and agarose (0.5 ml, 45 °C) were quickly instilled in the trachea. The lungs were immediately covered with ice for 2 min to solidify the agarose gel, and were then removed and incubated in 1 ml of DP for 45 min at room temperature. The lungs were subsequently transferred to a 60‐mm culture dish containing 7 ml of 4‐(2‐hydroxyethyl)‐1‐piperazineethanesulfonic acid‐buffered Dulbecco's modified Eagle's medium (DMEM) and 100 U/ml DNase I at 4 °C. The lung tissue was gently detached from the bronchi. The cell suspension was filtered through progressively smaller cell strainers (100 µm and 40 µm; BD Bioscience) and nylon gauze (20 µm, Millipore, Molsheim, France). The cells were collected by centrifugation at 130 g for 8 min (4 °C) and re‐suspended in 2 ml of DMEM without foetal bovine serum.
Purification of APTIIs from raw cell suspensions
The cells were incubated in 500 µg immunoglobulin G (IgG) (Sigma‐Aldrich, St. Louis, MO, USA) for 1 h, at 37 °C and 5% CO2. After incubation, the cells were re‐centrifuged. The resultant pellet was re‐suspended in DMEM with 10% foetal bovine serum, 36.6 mg/ml of l‐glutamine (Sigma), 100 µg/ml of streptomycin (Sigma), 100 U/ml of penicillin (Sigma) and 10 ng/ml of keratinocyte growth factor (KGF) (Sigma). This suspension was pre‐plated for a period not shorter than 4 h in order to eliminate fibroblasts. The purified APTIIs were plated in Matrigel (Sigma)/collagen (70 : 30 v/v) at 37 °C and 5% CO2 according to Rice et al. (2002). The medium was changed after 24 h, and every 48 h thereafter. After the enzymatic digestion with DP and the mechanical dissociation of the lungs, around 10.7 ± 5.5 × 106 cells were recovered per mouse (n = 97). IgG purification reduced the final concentration to 6.06 ± 2.3 × 106 cells, with a recovery of 91.04 ± 1.95%.
Preparation of IgG plates
Non irradiated plastic plates (for bacterial growth) were added with 100 µl IgG diluted in phosphate‐buffered saline (PBS), pH 7.3, with 0.1% bovine serum albumin. The plates were kept for 3 h at 25 °C. This solution was removed and the plates were washed five times with PBS (1 ml), and once with DMEM.
Collagen preparation
A solution‐containing type IV collagen was prepared according to Michalopoulos & Pitot (1975). Approximately 1 g of collagen fibres were obtained from two Wistar rats. The fibres were irradiated by ultraviolet light for 24 h and dissolved in 300 ml of a 1 : 1000 acetic acid solution in sterile water, and stirred for 24 h at 4 °C. The suspension was allowed to rest for 24 h at 4 °C, after which the supernatant was recovered.
A 10 ml collagen solution was vigorously mixed with DMEM (10%) and the pH was adjusted to 7.0 with NaOH. Seven milliliters of Matrigel were added to each 3 ml of the collagen solution. A volume of 250 µl was used in each plate. Collagen polymerization was carried out for 30 min. The supernatant was aspirated and the plates were dried immediately before use.
Cell viability
The cellular viability was determined by the dye exclusion method using the vital dye trypan blue at 0.4% in PBS, pH 7.4.
Osmium tetroxide/tannic acid staining
Cells cultured for 2 days were fixed for 15 min in 1.5% glutaraldehyde, washed with PBS and post‐fixed with 1% osmium tetroxide for 1 h, followed by enough volume of tannic acid (1%, pH 6.8) to cover the fixed cultures overnight. APTIIs were visually checked for morphology and stained for lamellar bodies, an APTII marker, in order to determine the cell type in culture (Fig. 2c). APTIIs were identified in cultures by the presence of dark, lamellate cytoplasmic inclusions. For quantification purposes, about 500 cells were counted and the results were expressed as percentage. Only attached and spread cells were quantified.
Figure 2.
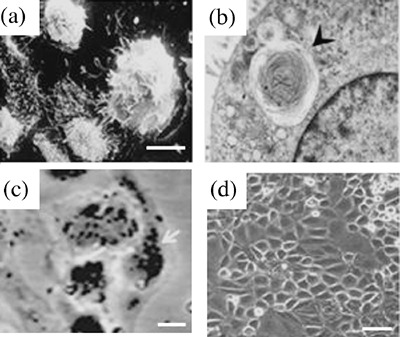
Morphological appearance of Alveolar Type II (APTII) cells in vitro. APTII cells were isolated from mouse lungs, and grown in culture to determine phenotype, as described in MATERIALS AND METHODS. (a) Scanning electron micrograph of APTII at day 2 of isolation. (b) Transmission electronmicrograph of APTII cells from Cx43+/– male mice, showing lamellar body in the cytoplasm (×25 600) (arrowhead). (c) Cells stained with tannic acid on day 2 of culture, APTII were densely packed and displayed distinct and numerous lamellar bodies (large dark granules) in > 95% of the cells in culture (arrowheads) in cells from Cx43+/– mice. (d) Phase contrast micrographs on day 4 representing cultured Cx43+/– and Cx43+/+ APTIIs. Bars, 10 µm (c) and 50 µm (d).
Electron microscopy
Cultures of APTII cells on 0.4 µm Millicell‐PC (Millipore) membranes were washed with PBS, and observed after 12 h of plating (Hasegawa et al. 2001). The cells were then fixed with glutaraldehyde 2.5% in PBS, post‐fixed in osmium tetroxide 1% in PBS, dehydrated in a graded ethanol series, and embedded in Epon. Blocks of interest were cut from the dishes and visualized by phase contrast. Microscopic sections for ultra‐structure analysis were contrasted with uranyl acetate for 10 min and observed in an electron microscope.
Rna extraction, cDNA construction and real‐time PCR analysis of Cx43 expression in lung
Total RNA was extracted from tissues by a single‐step technique with TRIzol Reagent (Invitrogen Life Technologies, Carlsbad, CA, USA) according to the manufacturer's protocol. The quality of the RNA samples was determined by electrophoresis in 2% agarose gels and staining with ethidium bromide. 18S and 28S bands were visualized under ultraviolet light (not shown). Total RNA was then treated with DNase I (Invitrogen Life Technologies) before processing further.
Oligo DT (1 µl) and dNTPs (1 µl) were added to the total RNA sample (500 µg) and incubated at 65 °C for 5 min. After that, Buffer 5X (Superscript II) (4 µl), DTT (1 m, 2 µl) and RNAse OUT (1 µl) were added, and the mixture was incubated at 42 °C for 2 min. Again Superscript II (1 µl) was added, followed by incubation at 42 °C for 50 min and subsequent incubation was performed at 70 °C for 15 min. For the removal of reminiscent RNA, 1 µl RNAse H was added, and the mixture incubated at 37 °C for 20 min. All reagents were purchased from Invitrogen Life Technologies.
Real‐time PCR analysis was performed in ABI Prism®. 7000 Sequence Detection Systems (Applied Biosystems, Foster City, CA, USA) using TaqMan Universal Master Mix (Applied Biosystems, Part number 4304437). PCR primers and the TaqMan probes for Cx43 and glyceraldehyde‐3‐phosphate dehydrogenase (GAPDH) quantification were selected using the Primer Express software programmes (Applied Biosystems) and checked by a basic local alignment search tool search of GenBank. The Cx43 forwards primer, 5′‐GTGCCGGCTTCACTTTCA TTAAG‐3′, and the Cx43 reverse primer, 3′‐CCAAGGCGCTCCAGTCA‐5′, were chosen to amplify a 65‐bp fragment. Primers to Cx43 were designed from separate exons (GenBank accession numbers L_10387 and L_10388 for forwards and reverse primers, respectively). The internal Cx43 TaqMan probe (FAM‐3′‐TCTGGGCACCTCTCTTT‐5′‐NFQ) was designed following the general rules outlined by the manufacturer. The GAPDH primers, 5′‐CATGGCCTTCCGTGTTCCTA‐3′ forward primer and 3′‐GCGGCACGTCAGAT CCA‐5′ reverse primer (55‐bp) (GenBank NM_008084), and the TaqMan probe VIC‐5′‐CCCCCAATGTGTCCGTC‐3′‐MGBNFQ were used as a housekeeping control. The TaqMan probes carried a 5′‐reporter dye 6‐carboxy‐fluorescein (FAM to Cxs) or (VIC for GAPDH), a 3′‐non fluorescent quencher dye (NFQ) and a minor groove binding (MGB). The primers and probes were used with 100% efficiency at the final concentrations of 0.9 µm and 0.25 µm, respectively.
The thermal cycling conditions to cDNA quantification assays were established according to ABI Prism® 7000 sequence detection systems parameters (Applied Biosystems). Analysis of relative gene expression data was performed according to the 2‐ΔΔCT method (Livak & Schmittgen 2001).
Cx43 immunostaining
For in vitro assays, 5 × 106/ml cells were seeded in eight‐well permanox slide (Laboratory‐Tek Brand Products, Naperville, IL, USA) and cultured for 4 days. The medium was removed and the cells were washed three times with PBS, pH 7.3 at 37 °C. Cells were permeabilized for 2 min with methanol : acetone 1 : 1 at room temperature. Then, slides were washed five times in PBS and blocked in block solution (PBS, 0.5% Triton X‐100, 2% heat‐inactivated goat serum) for 45 min. Monoclonal anti‐Cx43 diluted 1 : 100 (Zymed, San Francisco, CA, USA) was used.
Immunoblotting
Confluent cultures of APTII cells in 35‐mm dishes were washed with PBS and directly lysed in sodium dodecyl sulfate (SDS) sample buffer (60 mm Tris‐HCl, pH 6.8, 0.5% SDS). Protein extracts were subjected to SDS‐polyacrylamide gel electrophoresis and transferred to a nitrocellulose membrane. Non‐specific staining was blocked by 3% bovine serum albumin overnight and the blots were incubated with polyclonal rabbit anti‐Cx43 antibody (1/1000, 2 h) followed by incubation with a secondary peroxidase‐conjugated antirabbit antibody (1/500). A 3,3′‐diaminobenzidine solution was used to reveal Cx43 protein.
Studies of cell–cell communication capacity by scrape‐loading technique
Gap junctional intercellular communication capacity was assessed by the technique of scrape loading described by El‐Fouly & colleges (1987). Briefly, GJIC capacity was evaluated by the intercellular transfer of 5% Lucifer yellow (Sigma), in PBS after 5 min. The cells were fixed in 4% paraformaldehyde and photographs were taken with a 40X objective. Stained cells were counted in each side of rhodamine albumin in triplicate experiments.
Cell proliferation, measured by the MTT assay
APTIIs were plated in 35 mm dishes and the cell proliferation index was measured by reduction of the yellow dye 3‐(4,5‐dimethylthazol‐2‐yl)‐2,5‐diphenyltetrazolium bromide (MTT, Sigma), to a blue formazan product. Optical density of the blue formazan product was measured at 570 nm in a Shimadzu spectrophotometer. Triplicate experiments were performed.
Statistical analysis
All results were analyzed with a two‐tail Student's t‐test. Values of P < 0.05 were considered significant.
RESULTS
Characterization of APTII cells in culture
Morphology and presence of lamellar body were investigated to confirm the identity of APTIIs used in this study (Fig. 2a,b). About 95.9 ± 0.2% of isolated cells were typical APTIIs after osmium tetroxide staining (Fig. 2c). A total of 92.9 ± 4.0% of cells were viable by the trypan blue exclusion test. APTIIs used in this study showed characteristic morphology in a 4‐day culture (Fig. 2d).
Connexin localization in APTIIs
Immunostaining for Cx43 resulted in a punctuate pattern on the plasma membranes of both Cx43+/+ and Cx43+/– cells (Fig. 3a,b). However, the intensity was much higher in Cx43+/+ cells in comparison to Cx43+/– cells.
Figure 3.
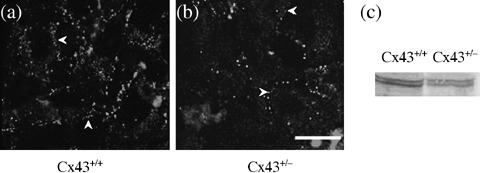
Localization of Cx43 in Alveolar Type II (APTII) cells by indirect immunofluorescence method on day 4 of culture. For the identification of Cx43‐expression in APTII cells from Cx43+/– and Cx43+/+, APTIIs were collected with Dispase, cultivated on glass slides for 4 days, permeabilized, immunostained with anti‐Cx43 (a and b) antiserum followed by rhodamine‐conjugated goat antirabbit immunoglobulin G (IgG) secondary antibody, and imaged in fluorescence microscopy. Negative control (APTII cells submitted to immunocytochemistry but omitting the primary antibody) not showed. Note that Cx43 was found in cell‐to‐cell region in both Cx43+/+ (a) and Cx43+/– (b) mice (arrowheads). Cx43 was higher in the membrane area of Cx43+/+ mice in comparison to Cx43+/– mice. These experiments were repeated twice for each plate with similar results. Bar, 10 µm. (c) Immunoblots of Cx43 in APTII cells. APTII cells cultured were solubilized, resolved by sodium dodecyl sulfate‐polyacrylamide gel electrophoresis, transferred to polyvinylidene difluoride, and blotted with anti‐Cx43. Kaleidoscope pre‐stained standards were used as loading control (not showed). For positive controls, HeLa cells transfected with the Cx43 gene was used (results not showed). Cx43 clones were kindly supplied by Dr Mesnil. These experiments were repeated twice for each Cx with similar results.
Expression of connexins by real‐time PCR and immunoblot analysis
Western blot was performed in 4‐day cultures of APTII. Cx43 is less expressed in Cx43+/– APTIIs than in Cx43+/+ APTIIs and showed a difference of 50% in relation to heterozygous mice (Table 1 and Fig. 3).
Table 1.
Levels of normalized Connexins mRNA in mouse lungs determined by quantitative RT‐PCR a
| Groups | Difference in cycles between Cx43 and GAPDH ΔCT | Normalized Cx43 amount 2‐ΔΔCT |
|---|---|---|
| Cx43+/+ | 6.7 ± 0.2 | 1.2 ± 0.03 |
| Cx43+/– | 8.1 ± 0.7 | 0.5 ± 0.04* |
Using reverse transcriptase, cDNA was synthesized from 500 µg of total RNA. Aliquots of cDNA were used as templates for real‐time PCR reactions containing primers and probes for Cx43, or primers and probe for GAPDH. Each reaction contained cDNA derived from 20 ng of total RNA. Eight different lungs from each group were analyzed, and four replicates of each reaction were performed by RT‐PCR.
P < 0.05, Tukey‐Kramer test.
Evaluation of APTII proliferation
Table 2 shows the proliferation of APTII cells at two culture periods. At both the time‐points, APTIIs from Cx43+/+ mice showed a lower proliferation rate in comparison to Cx43+/– cells (P < 0.05), quantified by the MTT‐test. At 8 days in culture, APTII cells reached confluence and started dying.
Table 2.
Growth curves representing MTT‐test of APTII cells from Cx43+/– and Cx43+/+ mice in primary culture
| Groups | Optical density | ||
|---|---|---|---|
| 4 Days | 6 Days | 8 Days | |
| Cx43+/+ | 0.660 ± 0.043 | 1.113 ± 0.042 | 0.964 ± 0.011 |
| Cx43+/– | 0.901 ± 0.010* | 1.588 ± 0.015* | 1.096 ± 0.018* |
Cells (5 × 104/ml) were plated in 35 mm dishes and cultured in conditioned medium, as described in METHODS. Results obtained from Cx43+/– mice were statistically significant in each culture periods. These results are represented as means from triplicate experiments. *Significant results (P < 0.05).
GJIC capacity in cultured APTII cells by the scrape‐loading technique
This technique was performed on the fourth day after APTII seeding. The amplitude of lucifer yellow diffusion after 5 min was 90 ± 8 in APTIIs from Cx43+/+ groups and 38 ± 6 in APTIIs from Cx43+/– groups (Fig. 4), representing a statistically significant difference (P < 0.05).
Figure 4.
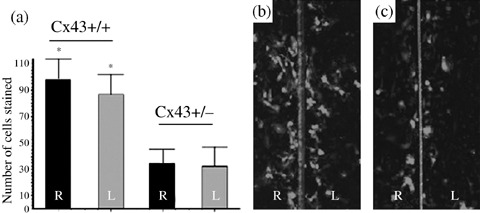
Gap junctional intercellular communication (GJIC) detected by the scrape loading and dye transfer (SLDT) method in Alveolar Type II (APTII) cells on day 4 of culture. Confluent monolayers were scraped with a sharp scalpel and loaded with Lucifer Yellow (LY, 457.25 kDa) (5% in PBS) for 5 min at room temperature. Observations were performed under epifluorescence. (a) Number of APTIIs from Cx43+/+ mice stained in green was counted in each side of the rhodamine‐albumin line (red, 10 kDa) used as a negative control. (b) APTIIs from Cx43+/+ mice. (c) APTIIs from Cx43+/– mice. Note in the centre lane that rhodamine‐albumin remains on the scraped area. These experiments were repeated three times for each plate with similar results. For each experiment, 107 APTII cells were plated initially. On day 4 of culture the cells reached more than 90% of confluence and the cells were scraped in region with complete confluence. *Represent significant results (P < 0.05).
DISCUSSION
It has been reported that chemically induced pulmonary adenomas originate from APTII cells (Mason et al. 2000). We have earlier reported the higher incidence of urethane‐induced lung adenomas in Cx43+/– mice compared to Cx43+/+ mice (Avanzo et al. 2004). In this study, we have examined the consequences of the deletion of one allele of Gja1 (Cx43 gene) for GJIC capacity and the associated alterations in the proliferation of APTIIs.
In order to accomplish this goal, we have first standardized the procedure to isolate these cells and maintain them in culture. It is known that maintenance and differentiation of alveolar cells in culture can be profoundly altered by both the soluble and insoluble macromolecules that compose the extracellular matrix, and the developmental fate of APTII cells can be altered by changing matrix or growth factor components (Isakson et al. 2001). We used a combination of three different methods to establish the primary culture from APTII mouse cells (Corti et al. 1996; Dobbs & Gonzalez 2002; Rice et al. 2002). The matrigel : collagen type IV matrix (70 : 30 v/v) plus Dulbecco's Modified Eagle's Medium (DMEM) 10/KGF allowed us to keep APTIIs in culture with an abundant presence of lamellar bodies, compared to a previous study that utilized type I collagen/fibronectin matrix supplemented with KGF (Isakson et al. 2001).
We also determined the presence of Cx43 in gap junction plaques in culture. On the basis of immunofluorescence studies and real‐time PCR analysis, Cx43 of APTIIs showed lower levels of expression in Cx43+/– compared to Cx43+/+ cells. These results were confirmed by immunoblotting and were in agreement with GJIC results. Our scrape‐loading study with lucifer yellow dye‐coupling showed that the GJIC capacity was lower in Cx43+/– derived APTII cells. This study of communication capacity is important, because it allows the quantification of functional parameters of gap junctions. Impaired GJIC has been shown to correlate with neoplastic transformation in several human tissues, such as breast (Laird et al. 1999), brain (Soroceanu et al. 2001), endometrium (Saito et al. 2001), prostate (Hossain et al. 1999) and lung (Ruch et al. 2001). This result is in accordance with our previous study showing that Cx43+/– mice are more susceptible to lung carcinogenesis. During Cx43 deficiency, adenoma cells from male or female Cx43+/– mice presented higher numbers of S‐phase cells than those from Cx43+/+ mice, suggesting that APTIIs are implicated in this phenomenon (Mason & Shannon 1997; Avanzo et al. 2004).
Several molecules can pass through gap junction channels and could directly influence the cell growth. After cell proliferation analyses using MTT‐test, we observed that isolated APTIIs cultured from Cx43+/– mice showed a higher proliferation rate in comparison to Cx43+/+ APTIIs. According to Loewenstein (1979) and Trosko & Ruch (1998), the communication‐deficient cells may not exchange neither inhibitory nor stimulatory factors, being unable to obey the homeostatic mechanisms present in its respective tissue and accordingly this may lead to deregulated cell proliferation.
In conclusion, this study shows, for the first time, that the deletion of a single Cx43 allele results in the reduction of GJIC capacity in APTIIs, which is associated with an increase in cell growth rate. Because chemically induced lung adenomas in mice are reportedly originate from APTIIs, these alterations may play a critical role in their susceptibility to lung carcinogenesis.
ACKNOWLEDGEMENTS
The authors would like to thank Drs I. Plaisance, J. C. Hervé, F. Duthé, L. Cronier and A. Malassiné (University of Poitiers) for their assistance. This work is part of the PhD thesis of J. L. Avanzo in the Experimental and Comparative Pathology Program of the Faculty of Veterinary Medicine and Animal Science of the University of São Paulo. This research was supported by grant 01/06820‐2 from Fundação de Amparo a Pesquisa do Estado de São Paulo (FAPESP). J. L. Avanzo was supported by a fellowship from FAPESP 01/06821‐9 and CAPES/COFECUB 386/02. This work is part of the International Cooperation Programme CAPES/COFECUB (Proc. 386/02).
REFERENCES
- Abraham V, Chou ML, Debolt KM, Koval M (1999) Phenotypic control of gap junctional communication by cultured alveolar epithelial cells. Am. J. Physiol. 276, L825–L834. [DOI] [PubMed] [Google Scholar]
- Avanzo JL, Mesnil M, Hernandez‐Blazquez FJ, Mackowiak II, Mori CM, Da Silva TC, Oloris SC, Garate AP, Massironi SM, Yamasaki H, Dagli ML (2004) Increased susceptibility to urethane‐induced lung tumors in mice with decreased expression of connexin43. Carcinogenesis 25, 1973–1982. [DOI] [PubMed] [Google Scholar]
- Charles AC, Merrill JE, Dirksen ER, Sanderson MJ (1991) Intercellular signaling in glial cells: calcium waves and oscillations in response to mechanical stimulation and glutamate. Neuron 6, 983–992. [DOI] [PubMed] [Google Scholar]
- Corti M, Brody AR, Harrison JH (1996) Isolation and primary culture of murine alveolar type II cells. Am. J. Respir. Cell Mol. Biol. 14, 309–315. [DOI] [PubMed] [Google Scholar]
- Dobbs LG, Gonzalez RF (2002) Isolation and culture of pulmonary alveolar epithelial type II cells In: Freshney RI, Freshney MG, eds. Culture of Epithelial Cells, p. 277 New York: Wiley‐Liss Inc. [Google Scholar]
- El‐Fouly MH, Trosko JE, Chang CC (1987) Scrape‐loading and dye transfer: a rapid and simple technique to study gap junctional intercellular communication. Exp. Cell Res. 168, 422–430. [DOI] [PubMed] [Google Scholar]
- Fehrenbach H (2001) Alveolar epithelial type II cell: defender of the alveolus revisited. Respir. Res. 2, 33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkbeiner S (1992) Calcium waves in astrocytes‐filling in the gaps. Neuron 8, 1101–1108. [DOI] [PubMed] [Google Scholar]
- Goodenough DA, Goliger JA, Paul DL (1996) Connexins, connexons and intercellular communication. Annu. Rev. Biochem. 65, 475–502. [DOI] [PubMed] [Google Scholar]
- Hasegawa S, Yamada K, Inoue H, Azuma N, Suzuki M, Matsuoka T (2001) Establishment of pulmonary alveolar type II cell line from p53‐deficient mice. Lung 179, 21–29. [DOI] [PubMed] [Google Scholar]
- Hennemann H, Dahl E, White JB, Schawarz HJ, Lalley PA, Chang S, Nicholson BJ, Willecke K (1992) Two gap junction genes, connexin 31.1 and 30.3, are closely linked on mouse chromosome 4 and preferentially expressed in skin. J. Biol. Chem. 267, 17225–17233. [PubMed] [Google Scholar]
- Hossain MZ, Jagdale AB, Ao P, Leciel C, Huang RP, Boynton AL (1999) Impaired expression and posttranslational processing of connexin 43 and down regulation of gap junctional communication in neoplastic human prostate cells. Prostate 38, 55–59. [DOI] [PubMed] [Google Scholar]
- Isakson BE, Lubman RL, Seedorf GJ, Boitano S (2001) Modulation of pulmonary alveolar type II cell phenotype and communication by extracellular matrix and KGF. Am. J. Physiol. Cell Physiol. 281, C1291–C1299. [DOI] [PubMed] [Google Scholar]
- Kasper M, Traub O, Relmann H, Grossmann H, Muller M, Wenzel KW (1996) Upregulation of gap junction protein connexin 43 in alveolar epithelial cells of rats with radiation‐induced pulmonary fibrosis. Histochem. Cell Biol. 106, 419–424. [DOI] [PubMed] [Google Scholar]
- King TJ, Lampe PD (2004) The gap junction protein connexin32 is a mouse lung tumor suppressor. Cancer Res. 64, 7191–7196. [DOI] [PubMed] [Google Scholar]
- Laird DW, Fistouris P, Batist G, Alpert L, Huynh HT, Carystinos GD, Alaoui‐Jamali MA (1999) Deficiency of connexin43 gap junctions is an independent marker for breast tumors. Cancer Res. 59, 4104–4110. [PubMed] [Google Scholar]
- Lee YC, Yellowley CE, Donahue HJ, Rannels DE (1997) Expression of functional gap junctions in cultured pulmonary alveolar epithelial cells. Am. J. Physiol. 272, L1105–L1114. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real‐time quantitative PCR and the 2‐ΔΔCT method. Methods 25, 402–408. [DOI] [PubMed] [Google Scholar]
- Loewenstein WR (1979) Junctional intercellular communication and the control of growth. Biochim. Biophys. Acta 560, 1–65. [DOI] [PubMed] [Google Scholar]
- Mason RJ, Kalina M, Nielsen LD, Malkinson AM, Shannon JM (2000) Surfactant protein C expression in urethane‐induced murine pulmonary tumors. Am. J. Pathol. 156, 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason RJ, Shannon JM (1997) Alveolar type II cells In: Crystal RG, West JB, Barnes JP, Weibel ER, eds. The Lung: Scientific Foundations, p. 543 Philadelphia, PA: Lippincott‐Raven. [Google Scholar]
- Mesnil M, Krutovskikh V, Piccoli C, Elfgang C, Traub O, Willecke K, Yamasaki H (1995) Negative growth control of HeLa cells by connexin genes: connexin species specificity. Cancer Res. 55, 629–639. [PubMed] [Google Scholar]
- Michalopoulos G, Pitot HC (1975) Primary culture of parenchymal liver cells on collagen membranes. Exp. Cell Res. 94, 70–78. [DOI] [PubMed] [Google Scholar]
- Ohshima M, Ward JM, Singh G, Katyal SL (1985) Immunocytochemical and morphological evidence for the origin of N‐nitrosomethylurea‐induced and naturally occurring primary lung tumors in F344/NCr rats. Cancer Res. 45, 2785–2792. [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J (1995) Cardiac malformation in neonatal mice lacking connexin43. Science 267, 1831–1834. [DOI] [PubMed] [Google Scholar]
- Rehm S, Ward JM, Ten Have‐Opbroek AA, Anderson LM, Singh G, Katyal SL, Rice JM (1988) Mouse papillary lung tumors transplacentally induced by N‐nitrosoethylurea: evidence for alveolar type II cell origin by comparative light microscopic, ultrastructural, and immunohistochemical studies. Cancer Res. 48, 148–160. [PubMed] [Google Scholar]
- Rice WR, Conkright JJ, Na CL, Ikegami M, Shannon JM, Weaver TE (2002) Maintenance of the mouse type II cell phenotype in vitro . Am. J. Physiol. Lung Cell Mol. Physiol. 283, L256–L264. [DOI] [PubMed] [Google Scholar]
- Ruch RJ, Porter S, Koffler LD, Dwyer‐Nield LD, Malkinson AM (2001) Defective gap junctional intercellular communication in lung cancer: loss of an important mediator of tissue homeostasis and phenotypic regulation. Exp. Lung Res. 27, 231–243. [DOI] [PubMed] [Google Scholar]
- Saez JC, Connor JA, Spray DC, Bennett MV (1989) Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5‐trisphosphate, and to calcium ions. Proc. Natl. Acad. Sci. USA 86, 2708–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Nishimura M, Kudo R., Yamasaki H (2001) Suppressed gap junctional intercellular communication in carcinogenesis of endometrium. Int. J. Cancer 93, 317–323. [DOI] [PubMed] [Google Scholar]
- Soroceanu L, Manning TJ Jr, Sontheiner H (2001) Reduced expression of connexin‐43 and functional gap junction coupling in human gliomas. Glia 33, 107–114. [DOI] [PubMed] [Google Scholar]
- Traub O, Eckert H, Lichtenberg‐Frate C, Elfgang B, Bastide KH, Scheidtmann DF, Hulser DF, Willecke K (1994) Immunochemical and electrophysiological characterization of murine connexin 40 and 43 in mouse tissues and transfected human cells. Eur. J. Cell Biol. 64, 101–112. [PubMed] [Google Scholar]
- Trosko JE, Ruch RJ (1998) Cell‐cell communication in carcinogenesis. Front. Biosci. 3, D208–D236. [DOI] [PubMed] [Google Scholar]
- Yamakage K, Omori Y, Piccoli C, Yamasaki H (1998) Growth control of 3T3 fibroblast cell line established from connexin 43‐deficient mice. Mol. Carcinog. 23, 121–128. [DOI] [PubMed] [Google Scholar]


