Figure 4.
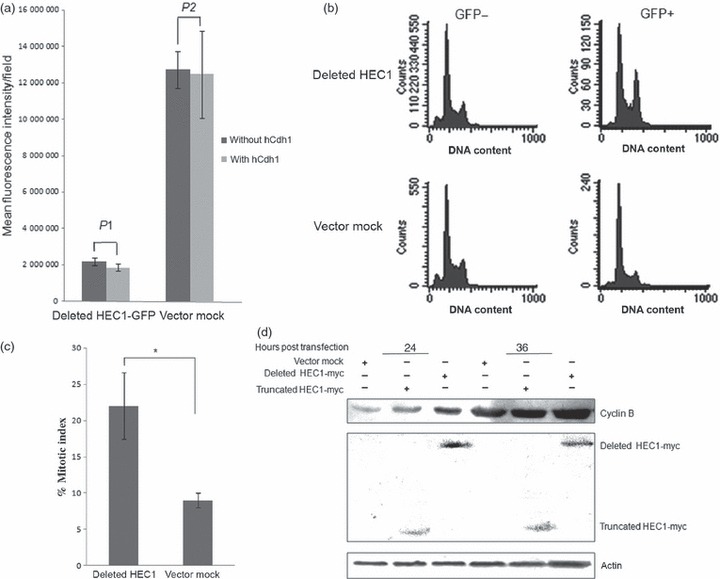
Accumulation of non‐degradable HEC1 results in mitotic arrest. (a) Deleted HEC1‐GFP failed to be degraded by APC/C‐Cdh1. The deleted mutant was inserted into pEGFP‐N1 (Above); this plasmid was co‐transfected with hCdh1 into HEK293T cells. Vector pEGFP‐N1 was used as a negative control. Protein levels of HEC were examined by monitoring fluorescence intensity of GFP (Bottom). P1 and P2 were both greater than 0.05 (P1 ≈ 0.45; P2 ≈ 0.85). (b) Overexpression of HEC1 variant could trigger G2/M delay in HEK293T. A significant increase in G2/M percentage was detected after GFP‐deleted HEC1 transfection. Cells were transfected with plasmids expressing GFP alone or GFP‐deleted HEC1. Cell cycle distribution was analysed according to DNA content of GFP‐positive cells at 36 h post‐transfection, using flow cytometry. (c) Cell population with mitotic chromatin increased in cells expressing deleted HEC1‐GFP protein. Cells transfected with plasmids encoding deleted HEC1‐GFP and GFP were stained by DAPI, at 24 h after transfection. Percentage of mitotic cells in each group was counted using a fluorescence microscope (P‐value is 0.03). (d) Overexpression of HEC1 variant could result in accumulation of cyclin B protein levels. An clear increase in cyclin B protein level was observed after deleted HEC1 transfection. Cells were transfected with plasmids encoding myc‐truncated HEC1, myc‐deleted HEC1 or pCMV‐myc. Cells were harvested and lysed to analyse cyclin B protein level at 24 and 36 h post‐transfection respectively.
