Abstract
Abstract. Objective: In our study, we describe the use of spider silk fibres as a new material in nerve tissue engineering, in a 20‐mm sciatic nerve defect in rats. Materials and methods: We compared isogenic nerve grafts to vein grafts with spider silk fibres, either alone or supplemented with Schwann cells, or Schwann cells and matrigel. Controls, consisting of veins and matrigel, were transplanted. After 6 months, regeneration was evaluated for clinical outcome, as well as for histological and morphometrical performance. Results: Nerve regeneration was achieved with isogenic nerve grafts as well as with all constructs, but not in the control group. Effective regeneration by isogenic nerve grafts and grafts containing spider silk was corroborated by diminished degeneration of the gastrocnemius muscle and by good histological evaluation results. Nerves stained for S‐100 and neurofilament indicated existence of Schwann cells and axonal re‐growth. Axons were aligned regularly and had a healthy appearance on ultrastructural examination. Interestingly, in contrast to recently published studies, we found that bridging an extensive gap by cell‐free constructs based on vein and spider silk was highly effective in nerve regeneration. Conclusion: We conclude that spider silk is a viable guiding material for Schwann cell migration and proliferation as well as for axonal re‐growth in a long‐distance model for peripheral nerve regeneration.
INTRODUCTION
Traumatic peripheral nerve injury is far more common than spinal cord injury, affecting 2.8% of all trauma patients (Chen et al. 2006). In Europe alone, 300 000 cases of peripheral nerve injury occur every year (Ciardelli & Chiono 2006). Consequences include long‐term disability, decreased functionality and major socio‐economic costs. Direct axonal repair is limited to short‐distance gaps and is additionally impaired by scarring and neuroma formation. Extensive nerve gaps must be reconstructed with structures creating a permissive environment for the axonal outgrowth. As the gold standard, current clinical practice makes use of autologous nerve grafts to compensate for lost and damaged nervous tissue, but availability of autologous nerve grafts is limited, and inevitably associated with loss of sensation at the donor site. Alternative approaches using allografts and consequent treatment with immunosuppressive pharmaceuticals can not be justified by the clinical outcomes (Evans et al. 2002).
Recent studies have focused on development of new therapeutic options derived from either natural or synthetic materials as nerve guiding material, but non‐resorbable polymers based on silicone and poly(tetrafluorethylene) tend to undergo fibrotic encapsulation. Moreover, late loss of functional recovery occurs, caused by progressive myelination and the resulting compression of axons within the conduits (Lundborg et al. 1994; Stanec & Stanec 1998; Lietz et al. 2005). Any substrate employed must not exert toxic effects on the neuronal cells nor evoke fibrotic or immune responses.
Natural materials are favoured with regard to their reduced cytotoxicity and enhanced biocompatibility, but must be immunologically compatible (Evans et al. 2002). Autologous veins have been used in experimental and clinical evaluations as interposition grafts (Chiu et al. 1988; Chiu & Strauch 1990; Strauch et al. 2001; Fansa & Keilhoff 2004). Transplantation of Schwann cells has allowed nerve regeneration across a 6‐cm gap in a rabbit model (Strauch et al. 2001). Unfortunately, when using venous tissue, lack of endogenous structures in veins has resulted in impaired nerve regeneration (Fansa et al. 2001). Recent research has demonstrated that unstructured, irregular inner matrices such as collagen rather impair axonal regeneration than aid it (Stang et al. 2005), whereas regular alignment of structural components was advantageous.
An ideal nerve graft promotes migration of supporting cells such as Schwann cells by providing endoneural‐like structures, thus supporting axonal re‐growth. Recent studies have focused on directional guiding structures in the lumina of conduits, such as oriented collagen (Dubey et al. 1999). As filling any lumen reduces space available for axonal outgrowth and may impair nerve regeneration, efforts have been undertaken to develop micropatterning processes, which allow guidance of neurite outgrowth. Miller et al. use micropatterned poly(D,L‐lactic acid) films for directional growth of neurites (Miller et al. 2001, 2002). This group has been able to show that microgrooves with a dimension of 10 µm width resulted in ideal neurite guidance, in accordance with work by Schmalenberg and Uhrich, who have further demonstrated that smaller pattern widths in microcontact‐printing enhanced Schwann cell orientation (Schmalenberg & Uhrich 2005). While Schwann cell guiding on surgical suturing material, polyglactin‐woven filaments and polydioxanone monofilaments, resulted in good cell adherence on polydioxanone filaments, in vitro acidic by‐products of their degradation process led to impaired nerve regeneration (Shen et al. 2001). In order to substitute synthetic materials with biocompatible fibres, we have been able to show previously that spider silk fibres support adhesion and proliferation of Schwann cells in culture (Allmeling et al. 2006).
In contrast to silkworm silk (Bombyx mori), which evoked fibroblastic response when used as nerve suture material in a rabbit model (DeLee et al. 1977), spider silk is devoid of sericin glue‐like proteins, which have been shown to exert no influence on biocompatibility and immunological response (Altman et al. 2003). Here, this study was undertaken to evaluate suitability of spider silk as guiding material for nerve regeneration.
MATERIALS AND METHODS
We constructed nerve grafts from isogenic veins filled with spider silk fibres, which were either supplemented with isogenic Schwann cells or used cell‐free. Nerve grafts were used to bridge a 20‐mm gap in the sciatic nerve of rats. Nerve regeneration was assessed by post‐operative behaviour of the animals, nerve conductance, and the relation between ipsilateral and contralateral gastrocnemius muscles. Explanted nerve grafts were examined histologically by light microscopy, immunofluorescent staining for S‐100 and neurofilaments, toluidine blue staining and transmission electron microscopy.
Collection of spider silk
Spider silk fibres were collected from adult females of the genus Nephila kept at our local animal facility. The spiders were refrigerated for a few minutes and subsequently gently fixated with a compress. We mechanically pulled out the fibres with a spider silk winding machine after stimulation of the major ampullate gland, and the spider silk was collected on spools. On average, we gained 150 m length of silk per hour. The spider silk was harvested under sterile conditions and was used without further manipulation.
Nerve constructs
The inferior cava vein of isogenic donor rats was dissected and a length of 20 mm harvested and treated under sterile conditions. After flushing with phosphate‐buffered saline (PBS, PAA, Cölbe, Germany), veins were frozen in 10% dimethyl sulfoxide (DMSO, Merck, Darmstadt, Germany) in foetal calf serum (FCS, Biochrom, Germany) with a needle inside the lumen to prevent collapse. Shortly before usage, veins were thawed slowly and washed with PBS. The spider silk was folded to an appropriate length to fit into the constructs and was pulled through the isogenic veins with forceps. The conduits were either injected with matrigel (dilution 1 : 3 with serum‐free melanocyte growth medium, Promocell, Heidelberg, Germany) or were left untreated.
Adult rat Schwann cells were prepared from the sciatic nerves of isogenic adult rats. Epineurium and perineurium were stripped and the nerves were cut into small pieces. These were incubated in Dulbecco's modified Eagle's medium (DMEM/F12, PAA) supplemented with 20% FCS and 100 µg/mL penicillin and streptomycin (PAA) at 37 °C, 5% CO2. After a growth period of 3 weeks, the Schwann cells were enzymatically digested with Dispase (Roche, Grenzach‐Wyhlen, Germany) and were homogenised using a Pasteur pipette. Purification of the culture was achieved by seeding cells on to a glass surface for 2 h. Contaminating fibroblasts settled down on the glass in this period while Schwann cells remained in the supernatant. This was washed with PBS and used in the further experiments. Preparative studies showed nearly 95% purity of the culture was obtained. Samples of isolated Schwann cells were observed under an inverted phase contrast microscope (Zeiss, Göttingen, Germany) to detect typical Schwann cell morphology.
Cell‐seeded constructs were injected with 300 000–500 000 Schwann cells per construct. Control grafts were injected with matrigel (BD, Heidelberg, Germany) at a 1 : 3 dilution with serum‐free melanocyte growth medium alone.
In vivo examination of biocompatibility of nerve grafts with spider silk
All experimental procedures with animals were approved by the Lower Saxony District Government (Hanover, Germany), and were carried out according to the institutional guidelines. Adult Lewis rats weighing 250–330 g were used in all experiments. The rats were narcotized with Ketamin 50 mg/kg, intraperitoneally (WDT, Garbsen, Germany) and Rompun 5 mg/kg, intraperitoneally (BayerVital, Leverkusen, Germany). Right sciatic nerves were exposed through a muscle‐splitting incision, and 20 mm long sections of the nerves were removed, proximal to trifurcations of the nerves. Twenty‐five rats were divided into five experimental groups: the first group was grafted with an isogenic nerve (group 1). To this end, 20 mm of the sciatic nerve were dissected, cut out and re‐inserted reversely into the resulting nerve gap. The other groups were grafted with nerve constructs composed of isogenic veins supplemented with spider silk (group 2), spider silk and Schwann cells (group 3) and spider silk, Schwann cells and matrigel (group 4), respectively. Control animals were grafted with isogenic veins and matrigel alone (group 5). The groups are depicted in Table 1. All animals were observed and evaluated regularly for post‐operative behaviour.
Table 1.
Experimental groups
| Group numbers | Groups | Number of animals (n) |
|---|---|---|
| 1 | Isograft | 5 |
| 2 | Veins and spider silk | 5 |
| 3 | Veins, spider silk and Schwann cells | 5 |
| 4 | Veins, spider silk, Schwann cells and matrigel | 5 |
| 5 | Veins and matrigel | 5 |
After 6 months, the rats were anaesthetized with Ketamin 50 mg/kg, intraperitoneally (WDT) and Rompun 5 mg/kg, intraperitoneally (BayerVital). The response to nerve stimulation with a Grass nerve and muscle stimulator (SD9 square pulse stimulator, Astro Medical, Rodgau, Germany, 5 V, duration 2 ms, frequency 1 Hz, polarity normal) was assessed; nerve conduction was not measured. Gastrocnemius muscles were harvested bilaterally. The weight ratio was calculated by setting the contralateral gastrocnemius muscle to 100% and put into relation to the ipsilateral muscle. Weight ratio serves as an indirect measurement of muscle regeneration after nerve repair (Stang et al. 2005).
Histology
Proximal, middle and distal parts of the nerves and nerve constructs were fixed in 4% buffered formalin (Microm, Walldorf, Germany), dehydrated in ethanol (Merck, Darmstadt, Germany), embedded in paraffin wax and sections were cut by means of a rotary microtome (Microm; 4 µm sections). Sections were air‐dried, deparaffinised and treated with descending ethanol concentrations. Then, sections were stained with haematoxylin and eosin (Merck) and were examined under a Zeiss microscope (Göttingen, Germany). All histological procedures were performed following common laboratory protocols (Romeis 1989).
Immunofluorescence
In sections antigenicity was retrieved using citrate buffer, blocked with 5% FCS in buffered saline at room temperature for 1 h and stained with anti‐S‐100 antibody (rabbit polyclonal, #Z0311 DAKO, Hamburg, Germany) and neurofilament polyclonal antibody (rabbit polyclonal, #M0762 DAKO) at 1/400 dilution in PBS (PAA)/1% FCS at 37 °C for 1 h, followed by treatment with 1/400 dilution of Alexa Fluor 488 goat antirabbit secondary antibody IgG (approximate absorption: 495 nm and fluorescent emission: 519 nm, green fluorescent, Molecular Probes, Leiden, The Netherlands). Finally, DNA was stained with blue fluorescent DAPI (4′,6‐diamidino‐2‐phenylindoledihydrochloride, Invitrogen, CA, USA). Sections were examined under an inverted fluorescence microscope (Zeiss) equipped with the appropriate barrier filters. Micrographs were taken using an Axio Cam MRM (Zeiss) and were processed with AxioVision (version 4.4; Zeiss, Jena, Germany).
Toluidine blue staining and transmission electron microscopy
A distal portion of the nerves and nerve constructs was fixed by immersion in 2.5% glutaraldehyde (Polysciences, Warrington, PA, USA) in 0.1 M Na‐cacodylate (Merck‐Schuchardt, Hohenbrunn, Germany), pH 7.3 for 24 h and post‐fixed in 2% Na‐cacodylate‐buffered osmium tetroxide (Riedel‐de Häen, Seelze, Germany) for 1 h, serially ethanol‐dehydrated, and then infiltrated and embedded in Epon (Serva, Heidelberg, Germany).
The 1 µm thick sections were treated with toluidine blue solution (Merck) for 30 s at 80 °C to stain for light microscopy. Nerve cross sections were evaluated randomly, and the equal opportunity rule was respected in sampling for establishing myelinated axon counts. Axons were counted with computer‐assistance (AxioVision 4.6; Zeiss, Jena, Germany) across an area of 200 µm2.
Ultrathin sections (70 nm) were cut using an ultramicrotome (Reichert‐Jung, Bensheim, Germany), placed on 300‐mesh copper/rhodium grids, stained with 5% uranyl acetate (Merck) in 70% methanol for 5 min, dried, stained with lead citrate (Merck) for 5 min, and then were dried and examined on a Zeiss EM 10 CR transmission electron microscope.
Statistical analysis
All data are expressed as mean ± SEM (standard error of the mean). Differences between experimental groups were assessed using the paired student's t‐test. P < 0.05 was considered to be statistically significant. The level of significance was adjusted according to Bonferroni to P < 0.05/number of tests.
RESULTS
Implant construction and surgical outcome
Spider silk‐filled vein grafts were constructed after Allmeling et al. (2006), vein diameters being approximately 1.5 mm. Spider silk collected on a spool was unwound and oriented longitudinally in bundels to fill about one quarter of the vein grafts. Mean diameter of drag‐line spider silk from Nephila spec. is roughly 10 µm, and is not sticky. No complications occurred during surgery and all wounds healed without complication; animals showed no tendency towards automutilation. Two rats from group 1 developed foot ulcers, the animals of group 3 grew elongated claws but no other animals showed any sign of impairment of the musculoskeletal system.
After 6 months, all conduits were well integrated into the host tissue and viable neovascularization was observed (Fig. 1a). Macroscopic observation revealed distinctive gastrocnemius muscle degeneration in animals from group 5 when compared to the other groups (Fig. 1b,c). Ispilateral and contralateral muscle ratios of groups 1, 2 and 4 were significantly better than group 5 (group 1: P = 0.002; group 2: P = 0.0108; group 4: P = 0.005). Group 3 animals showed no significant difference compared to group 5 (P = 0.2491) (Fig. 1d).
Figure 1.
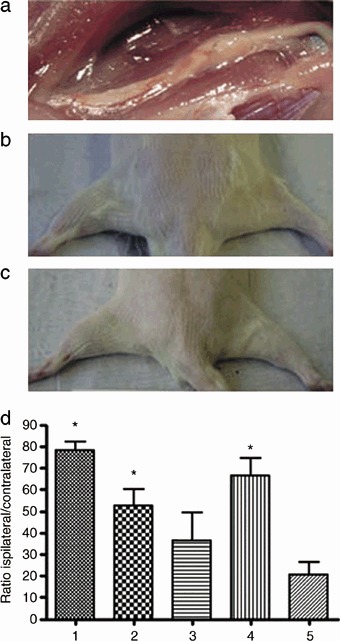
Macroscopic examination six months after nerve grafting. (a) Anatomic microdissection of the regenerated sciatic nerve. A 20 mm gap in the nerve had been interposed with an isogenic vein filled with spider silk fibres. The graft has maintained its original volume, is well integrated into the host tissue and well vascularized. (b) Comparison between the ipsilateral and contralateral gastrocnemius muscles in an animal grafted with veins and matrigel. On the left, the control is shown, compared to which the muscle on the right has atrophied following denervation. (c) Comparison between the ipsilateral and contralateral gastrocnemius in an animal grafted with veins and spider silk. On the left, the control is displayed, compared to which the muscle on the right has completely retained its mass. (d) Muscle to weight ratio of the ipsilateral and contralateral gastrocnemius muscles. Experimental groups are indicated according to Table 1. Data represent means ± SEM (n= 5). Statistical analysis was performed with paired student's t‐test and adjusted according to Bonferroni. Stars indicate significant differences (P < 0.05).
External stimulation of dissected grafts in vivo with a grass stimulator (5 V, duration 2 ms, frequency 1 Hz, polarity normal) resulted in different responses of the corresponding limbs. Best results were obtained in groups 1, 2 and 4 where stimulation resulted, in most cases, in contraction of the entire limb (group 1: four animals entire limb, one animal to the calf; group 2: four animals entire limb, one animal to the calf; group 4: five animals entire limb). In the groups 3 and 5, stimulation resulted in contraction to the knee or to the thigh (group 3: two animal to the toes, one animal to the knee, two animals no response; group 5: one animal to the toes, two animal to the calf, two animals no response).
Histological analysis
Histologically, it was shown that the walls of all conduits were penetrated by neovascularization but there were no signs of an inflammatory response or foreign body reaction (data not shown). In groups 1–4, longitudinal sections showed good regeneration of the sciatic nerve with nerve fibres and epineural and perineural sheaths (Fig. 2a–d) at the distal end. Neural tissue showed the undulating pattern typical of healthy nerves. Sections of group 5 animal tissues showed extensive void areas throughout the interponate (Fig. 2e).
Figure 2.
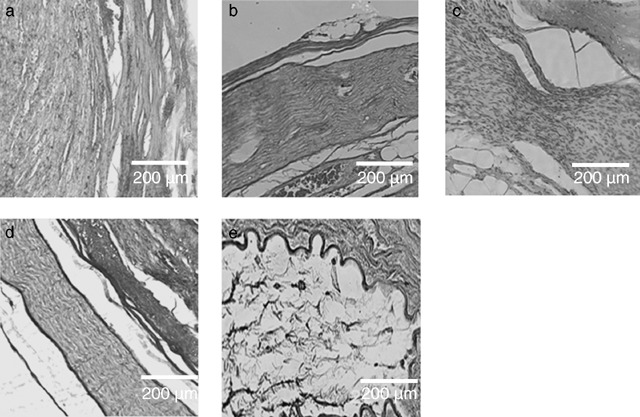
Comparative examination of the five experimental groups. Hamatoxylin‐eosin staining of paraffin‐embedded distal parts of the analyzed nerve grafts, harvested six months after implantation. Longitudinal sections were analyzed under a light microscope. The original magnification was 100×. (a) Isogenic nerve graft. Neural tissue is regularly patterned along the longitudinal axis. (b) Artificial nerve conduit consisting of vein and spider silk. The neural tissue is regularly patterned. Note the epineural and perineural sheath at the margin of the construct. (c) Artificial nerve conduit consisting of vein, spider silk and Schwann cells. (d) Artificial nerve conduit consisting of vein, spider silk, Schwann cells and matrigel. Axons are aligned along the longitudinal axis and epineural and perineural sheaths are visible. (e) Artificial nerve conduit consisting of vein and matrigel. Axonal regeneration is not visible, and no cells can be detected inside the conduit.
In nerve regeneration, Schwann cells perform important functions by secreting neurotrophic factors and guiding the regenerating axons (Chen et al. 2006). Since throughout the literature (Strauch 2000), supplementation of artificial nerve grafts with Schwann cells has resulted in better regeneration, especially in gaps larger than 10 mm, we examined Schwann cell distribution in the nerve grafts from groups 2 and 4. Similar results were obtained for constructs supplemented with cells (Fig. 3a) and for cell‐free constructs (Fig. 3b). Immunostaining for S‐100 showed good distribution along regenerated axons (Fig. 3a,b). This indicates that spider silk fibres successfully served as internal orientation structures promoting successful migration of host Schwann cells along the spider silk fibres. Sections stained without primary antibody only showed a faint and diffuse level of autofluorescence (Fig. 3c).
Figure 3.
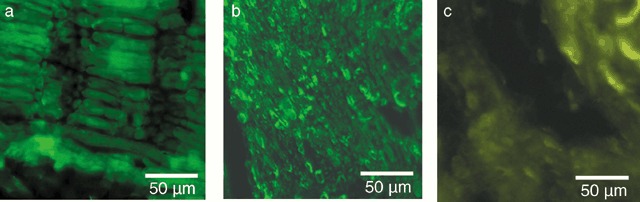
Remyelinisation of the artificial nerve conduits. The distal part of the nerve graft was paraffin‐embedded and processed for immunostaining with anti‐S100 followed by primary antibody detection with Alexa Fluor 488. Photomicrographs were taken at an original magnification of 400×. (a) Longitudinal section through a conduit consisting of vein, spider silk, Schwann cells and matrigel. Schwann cells stained positive alongside the neurites. (b) Longitudinal section through a conduit consisting of vein and spider silk. Schwann cells stained positive alongside the neurites, although the section was slightly misaligned with the longitudinal axis. Note that the cell‐free conduit was repopulated with Schwann cells until the distal end. (c) Negative control without primary antibody shows only a slight level of autofluorescence.
Toluidine blue‐stained sample cross sections indicated that the gap was filled with myelin sheaths of homogenous thickness, along the interponates (Fig. 4a–d). Smaller and larger axon diameters were equally distributed throughout the nerve. Once again, there was no obvious difference between the groups 1–4 (Fig. 4a–d) but a marked difference to group 5 (Fig. 4e). Images were assessed for presence of axons/myelin complexes in 200 µm2 cross sectional areas. Groups treated with spider silk showed histologically and morphometrically a significantly increased axon count compared to the group grafted with veins and matrigel alone (Fig. 4f).
Figure 4.
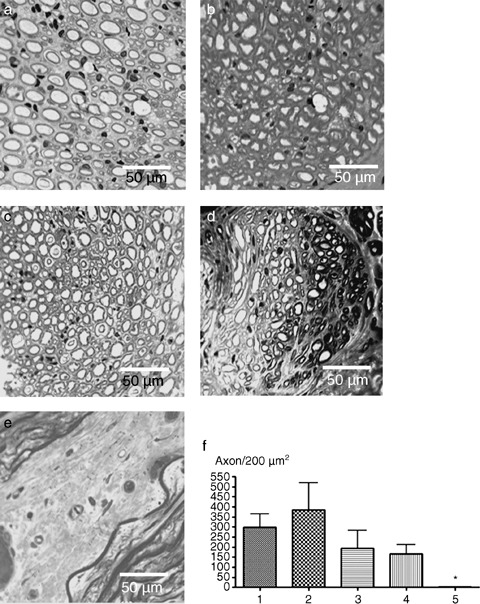
Toluidine‐blue‐stained cross‐section. (a–e) A distal part of the conduit was embedded in Epon and processed for Toluidine blue staining. Photomicrographs were taken at an original magnification of 400×. (a) Cross section through an isogenic nerve. This group showed a high number of axons and good remyelination. (b) Cross section through a conduit consisting of vein and spider silk. This group showed remyelinated axons with proper myelin sheaths and a fascicular structure. (c) Cross section through a conduit consisting of vein, spider silk and Schwann cells. The axons are evenly distributed throughout the conduit and encircled by myelin sheaths. (d) Cross section through a conduit consisting of vein, spider silk, Schwann cells and matrigel. An irregular organisation with somewhat lower numbers of axons was observed in the distal part of the regenerated nerve. (e) Cross section through a conduit originally consisting of vein and matrigel without Schwann cells and spider silk. This group showed no remyelinated axons. (f) Analysis of fibre counts. The images were assessed for the presence of axons/myelin complexes in 200 µm2 cross sectional areas. Data represent the mean + SEM (n= 5). Statistical analysis was performed with paired student's t‐test and adjusted according to Bonferroni. Stars indicate significant differences (P < 0.05).
Axonal regeneration along spider silk fibres was further examined by immunofluorescence studies for neurofilaments and by transmission electron microscopy. Interponates were positive for neurofilament proteins in longitudinal and cross sections (Fig. 5a,b). Bundles of neurites were densely packed, and had equally distributed diameters of healthy motor and sensory neurons. The axons traversed the whole length of the constructs, growing in correct orientation. Negative controls without primary antibody exhibited faint autofluorescence but without recognisable structures (Fig. 5c). By transmission electron microscopy, fully regenerated neuronal processes were visible (Fig. 5d). Inside electron‐dense myelin sheaths, neurites contained bundles of interfilaments, mitochondria and vesicles following microtubules. Spider silk fibres could not be detected anywhere.
Figure 5.
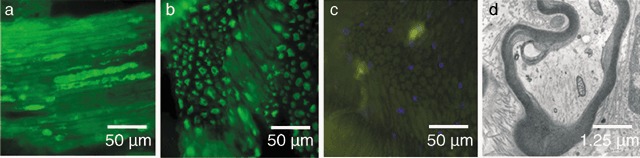
Axonal regeneration in a conduit consisting of vein, spider silk, Schwann cells and matrigel. (a–c) The distal part of the nerve graft was paraffin‐embedded and processed for immunostaining with anti‐neurofilament followed by primary antibody labelling with Alexa Fluor 488. Photomicrographs were taken at an original magnification of 400×. (a) Longitudinal section. Regenerated axons stained positive for neurofilament. The axons traverse the conduit in a regular pattern. (b) Cross section. The axons are arranged in a regular bundle. (c) Negative control without primary antibody shows only slight autofluorescence. Nuclei were stained with DAPI a nuclear labelling agent emitting blue fluorescence upon binding to AT regions of DNA. (d) A distal part of the conduit was embedded in Epon and processed for transmission electron microscopy. Photomicrograph was taken at an original magnification of 16000×. The image shows a cross section of a regenerated axon with bundles of interfilaments, mitochondria and vesicles following the microtubules. The axon is encircled by a myelin sheath.
DISCUSSION
Tissue‐engineering techniques seek to mimic structural and/or cellular organisation of autologous tissue. Consequently, artificial conduits have been developed using various biological or synthetic materials that have been used successfully to bridge small gaps in animal models and clinically. To date, no engineered graft has been found to be as effective as an autologous nerve graft (Johnson et al. 2005). While Schwann cell guiding along surgical stitching material, polyglactin‐woven filaments and polydioxanone monofilaments, resulted in good cell adherence on polydioxanone filaments, in vitro acidic by‐products of the degradation process led to impaired nerve regeneration (Shen et al. 2001). In order to substitute synthetic materials with biocompatible fibres, we have been able to show that spider silk fibres support adhesion and proliferation of Schwann cells in cell culture (Allmeling et al. 2006). In our study, we used a conduit of isogenic veins and spider silk to demonstrate the viability of spider silk fibres as a guiding material in peripheral nerve regeneration.
Spider silk is known for its unique properties such as high tensile strength, elasticity and toughness. It is a strong non‐elastic deformable, stretchable silk used in web formation. Major ampullate silk fibres from the species Nephila are composed of at least two spidroin proteins (MaSp1 and MaSp2) containing highly repetitive regions, which are composed mainly of glycine and alanine, but also contain tyrosine, glutamine and arginine residues (Sponner et al. 2005). MaSp1 is the dominant element of spider silk with a crystalline β‐sheet structure of repeated poly A or poly GA motifs alternating with GGX‐rich segments. This composition results in rigidity and tensile strength, but confers limited elasticity. MaSp2, however, is a stronger and more elastic element due to higher content of elastic β‐spirals built up by GPGXX motifs and a higher number of poly A repeats (Rising et al. 2005).
Spider silk as a bioengineering material does not swell, thereby avoiding deleterious neural compression (Sponner et al. 2005) and is of low immunogenicity (Vollrath et al. 2002). In our study, we found no evidence for any immunological reaction to the implanted nerve grafts. Spider silk is proteolytically degradable (Sponner et al. 2005), in contrast to bioresorbable polymers, which usually degrade with a switch to a non‐physiological pH (Ignatius & Claes 1996). Thus, spider silk is favourable for nerve regeneration, which is impaired in an acidic milieu. We found that degradation was complete after 6 months, without any apparent signs of cytotoxic by‐products and it has previously been demonstrated that spider silk has a permissive surface for cell adhesion (Allmeling et al. 2006). The sum of these factors provides a microenvironment suitable for nerve regeneration.
An important feature in the design of bioartificial conduits is the internal framework to support Schwann cell migration and proliferation, as well as axonal outgrowth. Recent literature describes the construction of artificial conduits with internal matrices consisting of longitudinally aligned fibres (Cai et al. 2005; Lietz et al. 2005; Stang et al. 2005; Ao et al. 2006) and our study further supports these observations by demonstrating that longitudinally aligned spider silk is well suited to guide migration of Schwann cells along the fibres, promoting and directing axonal outgrowth.
For larger defects, introduction of cells with regenerative capacity, such as Schwann cells, into the conduits has been shown to be beneficial. Implementation of Schwann cells allowed nerve regeneration through a 6‐cm gap in a rabbit model (Strauch et al. 2001). Other studies on bioartificial nerve conduits have confirmed the increase in nerve regeneration following addition of Schwann cells. It has been found that increased numbers of grafted Schwann cells results in a higher number of in myelinated axons (Guenard et al. 1992). In a study by Rutkowski and Heath (2002), a high density of supplemented Schwann cells led to increased numbers of axons, however, indicating branching of axons, which resulted in mismatches in the periphery, and can lead to painful neuroma formation. Whereas earlier studies using a single thick thread of any sort as a conduit, found only as much as 10 axons per 200 µm2 (Shen et al. 2001), we have been able to show that using several thin spider silk threads leads to formation of up to 300 myelinated axons. Thus, if branching of the axon is inhibited by the conduit, likelihood for re‐innervation is greatly increased.
Interestingly, conduits containing spider silk fibres and Schwann cells alone resulted in inconsistent regeneration. While three animals showed convincing regeneration with strong results in all assessed areas, two animals failed to regenerate at all. We were not able to determine whether the impaired regeneration was caused by cell death of grafted Schwann cells or by poor stability due to lack of primary extracellular matrix. Frisch and Francis (1994) reported that a form of apoptosis termed ‘anoikis’ can be induced by lack of contact to extracellular matrix molecules. Examples of cell death caused by anoikis have been described for the central nervous system (Abe et al. 2004), and for Schwann cells grown on different extracellular matrix molecules (Weiner et al. 2001) also. Moreover, we cannot confirm the results of Donizelli (Donizelli et al. 2006), who found that grafts of vein and matrigel alone promote neural regeneration. In contrast to their findings, we discovered next to no myelinated nerve fibres in the corresponding group in our study. Our results suggest that successful nerve regeneration requires either supplementation of the veins with spider silk fibres, Schwann cells and matrigel, or use of veins with spider silk alone in order to promote migration of autologous cells.
Use of autologous veins has proved to be useful for nerve repair in several studies, especially when supplemented with Schwann cells and other internal structures (e.g. acellularized muscle; Fansa et al. 2001; Strauch et al. 2001; Geuna et al. 2003; Raimondo et al. 2005). Porosity has been demonstrated to be of greater importance than wall thickness by allowing influx of nutrients into the lumen of the construct while retaining growth factors secreted by the supporting Schwann cells (Rutkowski & Heath 2002). We were able to demonstrate that spider silk fibres served as ideal internal structures for nerve regeneration, supporting axonal outgrowth as well as Schwann cell migration. Constructs containing spider silk fibres and Schwann cells in a matrigel suspension and constructs containing spider silk fibres alone performed at least as well as autologous grafts under the chosen experimental conditions. In contrast to published studies working with models of large peripheral nerve lesions (Strauch et al. 2001), regeneration was achievable for both cellularized and cell‐free constructs.
Clinical relevance of our findings is further underscored by the fact that effective constructs can be crafted from veins and spider silk fibres alone. This results in minimal donor site morbidity as well as good immunological tolerance. As a consequence, we consider spider silk fibres to be a suitable and promising filling for peripheral nerve conduits in nerve tissue engineering.
ACKNOWLEDGEMENTS
We would like to thank A. Lazaridis and S. Jahn for their technical assistance. We are grateful to Daniel Weiner and William F. Burke for help with the manuscript. The work was funded by the Jung‐Stiftung, Hamburg, Germany.
REFERENCES
- Abe Y, Yamamoto T, Sugiyama Y, Watanabe T, Saito N, Kayama H, Kumagai T (2004) ‘Anoikis’ of oligodendrocytes induced by Wallerian degeneration: ultrastructural observations. J. Neurotrauma 21, 119–124. [DOI] [PubMed] [Google Scholar]
- Allmeling C, Jokuszies A, Reimers K, Kall S, Vogt PM (2006) Use of spider silk fibres as an innovative material in a biocompatible artificial nerve conduit. J. Cell. Mol. Med. 10, 770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, Lu H, Richmond J, Kaplan DL (2003) Silk‐based biomaterials. Biomaterials 24, 401–416. [DOI] [PubMed] [Google Scholar]
- Ao Q, Wang A, Cao W, Zhang L, Kong L, He Q, Gong Y, Zhang X (2006) Manufacture of multimicrotubule chitosan nerve conduits with novel molds and characterization in vitro . J. Biomed. Mater. Res. A 77, 11–18. [DOI] [PubMed] [Google Scholar]
- Cai J, Peng X, Nelson KD, Eberhart R, Smith GM (2005) Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J. Biomed. Mater. Res. A 75, 374–386. [DOI] [PubMed] [Google Scholar]
- Chen MB, Zhang F, Lineaweaver WC (2006) Luminal fillers in nerve conduits for peripheral nerve repair. Ann. Plast. Surg. 57, 462–471. [DOI] [PubMed] [Google Scholar]
- Chiu DT, Strauch B (1990) A prospective clinical evaluation of autogenous vein grafts used as a nerve conduit for distal sensory nerve defects of 3 cm or less. Plast. Reconstr. Surg. 86, 928–934. [DOI] [PubMed] [Google Scholar]
- Chiu DT, Lovelace RE, Yu LT, Wolff M, Stengel S, Middleton L, Janecka IP, Krizek TJ (1988) Comparative electrophysiologic evaluation of nerve grafts and autogenous vein grafts as nerve conduits: an experimental study. J. Reconstr. Microsurg. 4, 303–302. [DOI] [PubMed] [Google Scholar]
- Ciardelli G, Chiono V (2006) Materials for peripheral nerve regeneration. Macromol. Biosci. 6, 13–26. [DOI] [PubMed] [Google Scholar]
- DeLee JC, Smith MT, Green DP (1977) The reaction of nerve tissue to various suture materials: a study in rabbits. J. Hand Surg. [Am.]. 2, 38–43. [DOI] [PubMed] [Google Scholar]
- Donizelli R, Maiuri F, Piscopo GA, De Notaris M, Colella A, Divitiis E (2006) Role of extracellular matrix components in facial nerve regeneration: an experimental study. Neurol. Res. 28, 794–801. [DOI] [PubMed] [Google Scholar]
- Dubey N, Letourneau PC, Tranquillo RT (1999) Guided neurite elongation and schwann cell invasion into magnetically aligned collagen in simulated peripheral nerve regeneration. Exp. Neurol. 158, 338–350. [DOI] [PubMed] [Google Scholar]
- Evans GR, Brandt K, Katz S, Chauvin P, Otto L, Bogle M, Wang B, Meszlenyi RK, Lu L, Mikos AG, Patrick CW Jr (2002) Bioactive poly (1‐lactic acid) conduits seeded with Schwann cells for peripheral nerve regeneration. Biomaterials 23, 841–848. [DOI] [PubMed] [Google Scholar]
- Fansa H, Keilhoff G (2004) Comparison of different biogenic matrices seeded with cultured Schwann cells for bridging peripheral nerve defects. Neurol. Res. 26, 167–173. [DOI] [PubMed] [Google Scholar]
- Fansa H, Keilhoff G, Wolf G, Schneider W (2001) Tissue engineering of peripheral nerves: a comparison of venous and acellular muscle grafts with cultured Schwann cells. Plast. Reconstr. Surg. 107, 485–494. [DOI] [PubMed] [Google Scholar]
- Frisch SM, Francis H (1994) Disruption of epithelial cell–matrix interactions induces apoptosis. J. Cell Biol. 124, 619–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geuna S, Raimondo S, Nicolino S, Boux E, Fornaro M, Tos P, Battiston B, Perroteau I (2003) Schwann‐cell proliferation in muscle‐vein combined conduits for bridging rat sciatic nerve defects. J. Reconstr. Microsurg. 19, 119–123. [DOI] [PubMed] [Google Scholar]
- Guenard V, Kleitman N, Morrissey TK, Bunge RP, Aebischer P (1992) Syngeneic Schwann cells derived from adult nerves seeded in semipermeable guidance channels enhance peripheral nerve regeneration. J. Neurosci. 12, 3310–3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignatius AA, Claes LE (1996) In vitro biocompatibility of bioresorbable polymers: poly(L, DL‐lactide) and poly(L‐lactide‐co‐glycolide). Biomaterials 17, 831–839. [DOI] [PubMed] [Google Scholar]
- Johnson EO, Zoubos AB, Soucacos PN (2005) Regeneration and repair of peripheral nerves. Injury 36(Suppl. 4), S24–S29. [DOI] [PubMed] [Google Scholar]
- Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B (2005) Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials 8, 1425–1436. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rosen B, Abrahamson SO, Dahlin L, Danielsen N (1994) Tubular repair of the median nerve in the human forearm. Preliminary findings. J. Hand Surg. [Br.] 19, 273–276. [DOI] [PubMed] [Google Scholar]
- Miller C, Jeftinija S, Mallapragada S (2001) Micropatterned Schwann cell‐seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 7, 705–715. [DOI] [PubMed] [Google Scholar]
- Miller C, Jeftinija S, Mallapragada S (2002) Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 8, 367–378. [DOI] [PubMed] [Google Scholar]
- Raimondo S, Nicolino S, Tos P, Battiston B, Giacobini‐Robecchi MG, Perroteau I, Geuna S (2005) Schwann cell behavior after nerve repair by means of tissue‐engineered muscle‐vein combined guides. J. Comp. Neurol. 489, 249–259. [DOI] [PubMed] [Google Scholar]
- Rising A, Nimmervoll H, Grip S, Fernandez‐Arias A, Storckenfeldt E, Knight DP, Vollrath F, Engstrom W (2005) Spider silk proteins – mechanical property and gene sequence. Zool. Sci. 22, 273–281. [DOI] [PubMed] [Google Scholar]
- Romeis B (1989) Mikroskopische Technik. Urban & Schwarzenberg, München, Germany. [Google Scholar]
- Rutkowski GE, Heath CA (2002) Development of a bioartificial nerve graft. II. Nerve regeneration in vitro . Biotechnol. Prog. 18, 373–379. [DOI] [PubMed] [Google Scholar]
- Schmalenberg KE, Uhrich KE (2005) Micropatterned polymer substrates control alignment of proliferating Schwann cells to direct neuronal regeneration. Biomaterials 26, 1423–1430. [DOI] [PubMed] [Google Scholar]
- Shen ZL, Berger A, Hierner R, Allmeling C, Ungewickell E, Walter GF (2001) A Schwann cell‐seeded intrinsic framework and its satisfactory biocompatibility for a bioartificial nerve graft. Microsurgery 21, 6–11. [DOI] [PubMed] [Google Scholar]
- Sponner A, Schlott B, Vollrath F, Unger E, Grosse F, Weisshart K (2005) Characterization of the protein components of Nephila clavipes dragline silk. Biochemistry 44, 4727–4736. [DOI] [PubMed] [Google Scholar]
- Stanec S, Stanec Z (1998) Reconstruction of upper‐extremity peripheral‐nerve injuries with ePTFE conduits. J. Reconstr. Microsurg. 14, 227–232. [DOI] [PubMed] [Google Scholar]
- Stang F, Fansa H, Wolf G, Reppin M, Keilhoff G (2005) Structural parameters of collagen nerve grafts influence peripheral nerve regeneration. Biomaterials 26, 3083–3091. [DOI] [PubMed] [Google Scholar]
- Strauch B (2000) Use of nerve conduits in peripheral nerve repair. Hand Clin. 16, 123–130. [PubMed] [Google Scholar]
- Strauch B, Rodriguez DM, Diaz J, Yu HL, Kaplan G, Weinstein DE (2001) Autologous Schwann cells drive regeneration through a 6‐cm autogenous venous nerve conduit. J. Reconstr. Microsurg. 17, 589–595. [DOI] [PubMed] [Google Scholar]
- Vollrath F, Barth P, Basedow A, Engstrom W, List H (2002) Local tolerance to spider silks and protein polymers in vivo . In Vivo 16, 229–234. [PubMed] [Google Scholar]
- Weiner JA, Fukushima N, Contos JJ, Scherer SS, Chun J (2001) Regulation of Schwann cell morphology and adhesion by receptor‐mediated lysophosphatidic acid signaling. J. Neurosci. 21, 7069–7078. [DOI] [PMC free article] [PubMed] [Google Scholar]


