Abstract
Objectives
Human umbilical cord can be obtained easily and it represents a non‐controversial source of mesenchymal stem cells (MSCs) and umbilical cord Wharton's jelly‐derived MSCs (UC‐MSCs) have low immunogenicity. In this study, UC‐MSCs were induced to become steroidogenic cells and compared to bone marrow‐derived MSCs (BM‐MSCs).
Material and methods
UC‐MSCs and BM‐MSCs were induced to differentiate into steroidogenic cells by infection with adenovirus containing SF‐1. Expression of steroidogenic mRNA was analysed by real‐time RT‐PCR and steroid secretion was detected by ELISA testing. Viability of differentiated cells was examined using cell counting kit‐8 assay.
Results
Both UC‐MSCs and BM‐MSCs expressed typical MSC markers and could differentiate into adipocytes, osteocytes and chondrocytes and both cell types had the potential to differentiate into steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA. However, UC‐MSCs had significantly higher proliferative potential than BM‐MSCs and differentiated UC‐MSCs had significantly higher expression of all steroidogenic mRNAs tested over those of differentiated BM‐MSCs; this included P450 side‐chain cleavage enzyme, 3β‐HSD, 17β‐HSD type 3, LH‐R, ACTH‐R, P450c21 and CYP17. In addition, differentiated UC‐MSCs secreted significantly more steroidogenic hormones than differentiated BM‐MSCs, including testosterone and cortisol. Furthermore, differentiated UC‐MSCs had significantly higher cell viability than differentiated BM‐MSCs.
Conclusions
UC‐MSCs had significantly higher potential of steroidogenic differentiation than BM‐MSCs; thus, UC‐MSCs could be favourable cells of choice for cell‐based therapy for steroidogenic insufficiency compared to BM‐MSCs.
Introduction
Mesenchymal stem cells (MSCs) can self‐renew and differentiate into distinct multiple cell lineages 1. They can be isolated from different tissues, including bone marrow, adipose tissue and umbilical cord blood 2. MSCs isolated from bone marrow (BM‐MSCs) have been extensively studied while MSCs isolated from umbilical cord Wharton's jelly (UC‐MSCs) represent a more recently examined source of MSCs. Previous studies have shown that UC‐MSCs can differentiate into osteogenic, chondrogenic and adipogenic lineages and many other types of cells 3, 4, 5. In vivo transplantation experimentation has shown that transplantation of human UC‐MSCs into patients with severe and refractory systemic lupus erythematosus has had profound therapeutic effects even without use of immunosuppressive drugs 6. Transplantation of pig UC‐MSCs into rat brain have not suffered from immune rejection nor teratoma formation, up to 8 weeks after transplantation as xenografts, without need for immune suppression. Donor cells have stained positively with neurofilament antibody, suggesting that pig UC‐MSCs could differentiate into neurons or neuron precursors in vivo 7. Other studies have demonstrated that UC‐MSCs could be immunologically suppressive in mixed lymphocyte assays, inhibited T‐cell proliferation and had low immunogenicity 8. Thus, UC‐MSCs are gaining more attention as an ideal clinical source of MSCs for cell therapy and tissue engineering, due to their easy accessibility, high levels of proliferation, multipotency and low immunogenicity.
As men age, their serum testosterone levels fall to abnormal levels resulting in hypogonadism; this disease is termed partial androgen deficiency of the aging male (PADAM) and PADAM patients also suffer from further diseases of bone, muscle, brain and cardiovascular systems due to testosterone deficiency 9, 10. Testosterone supplementation is currently the main method of treatment of PADAM to increase testosterone levels and provide testosterone function. However, this can have a range of side‐effects and can cause many health problems, including inducing prostate cancer 11, 12, 13, 14, 15. Cell transplantation could be a future direction for treatment of this disease.
Leydig cells are derived from testis; these secrete 95% of the body's testosterone, the remaining 5% being secreted by adrenal cells. There are four types of Leydig cell involved in development: Leydig stem cells, Leydig progenitor cells, immature Leydig cells and adult Leydig cells 16. Leydig stem cells do not express Leydig cell lineage‐specific markers and do not secrete testosterone but Leydig progenitor cells, immature Leydig cells and adult Leydig cells all express Leydig cell lineage‐specific markers, including P450 side‐chain cleavage enzyme (P450scc), 3β‐hydroxysteroid dehydrogenase (3β‐HSD), cytochrome P450 17α‐hydroxylase (P450c17) and luteinizing hormone receptor (LH‐R). Leydig progenitor cells and immature Leydig cells secrete only limited amounts of testosterone, thus adult Leydig cells secrete the greatest quantity of testosterone among the four cell types 17, 18, 19. ACTH‐R is expressed in the adrenal gland and can induce adrenal cells to synthesize cortisol 20; expression of ACTH‐R is up‐regulated by ACTH and P450c21 is expressed only in the adrenal gland 21.
Steroidogenic factor 1 (SF‐1) is a member of a nuclear receptor family and is a key transcription factor for adrenal and gonadal development 22 and postnatal mice of SF‐1 knockout parents die due to adrenal and gonadal agenesis 23. Gonads and adrenal glands are the primary steroidogenic organs in mammals; testosterone is primarily secreted by Leydig cells of the testis of males and ovaries of females, and only small amounts are produced by adrenal glands. Previous studies have shown that BM‐MSCs and embryonic stem (ES) cells have differentiated into steroidogenic cells using SF‐1 24, 25, 26 administration. Due to limitations of use of human BM‐MSCs and ES cells for therapeutic applications, alternative sources of cells are required. In this study, UC‐MSCs and BM‐MSCs were infected with adenovirus containing SF‐1 and thus induced to differentiate into steroidogenic cells. Those from UC‐MSCs were compared to those from BM‐MSCs to determine their potential for steroidogenic differentiation.
Materials and methods
Isolation and culture of UC‐MSCs
Human umbilical cord was aseptically collected from full‐term caesarean section patients with their consent at the First Affiliated Hospital of Shantou University Medical College, Shantou, China. UC‐MSC isolation was performed as previously described 3. Umbilical cord Wharton's jelly was cut into 2–3 mm3 pieces which were cultured in a 37 °C incubator in six‐well plates (Corning Inc., Acton, MA, USA) containing growth medium prepared from Dulbecco's modified Eagle's medium‐low glucose (DMEM‐LG; Invitrogen, Carlsbad, CA, USA), supplemented with 10% (v/v) foetal bovine serum (FBS), 2 mm l‐glutamine, 5 ng/ml basic fibroblast growth factor (Invitrogen), 100 U/ml penicillin and 100 mg/ml streptomycin. Cells were harvested using 0.25% trypsin and medium was changed every 3 days.
Isolation and culture of BM‐MSCs
Human bone marrow samples were collected from donors (5‐ to 36‐years‐old) carrying no metabolic disease, and with full consent, at the First Affiliated Hospital of Shantou University Medical College, Shantou, China. BM‐MSCs were isolated as previously described 27. Briefly, bone marrow samples were layered over a lymphoprep gradient and centrifuged at 900 g for 15 min at room temperature. Isolated mononuclear cells were washed twice in Hank's solution, then resuspended in growth medium as described above, at 1–6 × 106 cells/cm2 density in six‐well plates (Corning Inc.). Cells were cultured in a 37 °C incubator, saturated humidity and 5% CO2. Medium was replaced after 3 days culture and non‐adherent cells were removed; medium was changed twice a week. At approximately 90% confluence, cells were harvested using 0.25% trypsin (Invitrogen) and passaged for expansion.
Identification of MSC marker expression by flow cytometry
Flow cytometric analysis was performed as previously described with modifications 28. Cells were harvested using 0.25% trypsin, washed in phosphate‐buffered saline (PBS) and incubated for 45 min at 4 °C in the dark, with the following anti‐human antibodies, conjugated to fluorescein isothiocyanate (FITC) or phycoerythrin (PE), anti‐: CD105‐PE, CD73‐PE, CD90‐PE, CD45‐PE, CD34‐FITC, CD14‐FITC, CD19‐PE and HLA‐DR‐FITC (BD Pharmingen, San Diego, CA, USA). PE‐conjugated IgG1 and FITC‐conjugated IgG1 were used as isotype controls (BD Pharmingen). After washing in PBS, specific fluorescence of 1 × 106 cells was analysed using a FACScan flow cytometer (Epics XL; Beckman Coulter, Miami, FL, USA).
Adipogenic differentiation of UC‐MSCs and BM‐MSCs
UC‐MSCs and BM‐MSCs were induced into adipogenic differentiation as previously described 2. Briefly, cells were harvested and cultured in 24‐well plates at 1 × 104 cells/cm2 density. After cells reached 70–80% confluence, growth medium was changed to adipogenic differentiation medium consisting of DMEM‐LG supplemented with 10% (v/v) FBS, 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 60 μm indomethacin, 1 μm dexamethasone, 0.5 mm 3‐isobutyl‐1‐methylxanthine (IBMX) and 5 μg/ml insulin solution (Sigma, St Louis, MO, USA); medium was changed every 3 days. After 21 days induction, generation of neutral lipid droplets was examined by staining with oil red O (Sigma); As control, cells were incubated in growth medium alone.
Osteogenic differentiation of UC‐MSCs and BM‐MSCs
Cells were induced into osteogenic differentiation as previously described, with modifications 3. Briefly, cells were harvested and cultured in 24‐well plates at 1 × 104 cells/cm2 density. After reaching 70–80% confluence, growth medium was changed to osteogenic differentiation medium consisting of DMEM‐LG supplemented with 10% (v/v) FBS, 2 mm l‐glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 100 nm dexamethasone, 0.2 mm l‐ascorbate and 10 mm β‐glycerophosphate (Sigma). Cells were incubated in osteogenic differentiation medium for 21 days and medium was changed every 3 days; as control, cells were incubated in growth medium only.
Osteogenic differentiation was examined by staining for presence of alkaline phosphatase (ALP), using a leucocyte alkaline phosphatase kit (kit 85L‐3R; Sigma) following manufacturer's instructions. Mineralization of osteogenic differentiation was detected using von Kossa staining according to the protocol, as previously described with modifications 3. Cells were fixed in 10% formalin (Sigma) for 15 min and stained with 5% silver nitrate (Sigma) for 15 min. Staining was developed by incubating cells in 1% pyrogallol (Sigma) and fixing in 5% sodium thiosulphate (Sigma) for 5 min.
Chondrogenic differentiation of UC‐MSCs and BM‐MSCs
Cells were induced into chondrogenic differentiation as previously described 2. To induce chondrogenic differentiation, cells were transferred to 15 ml polypropylene tubes and centrifuged at 500 g for 5 min, to form cell pellets at bottoms of tubes. Cells were cultured for 21 days in chondrogenic differentiation medium containing DMEM‐HG supplemented with 1% (v/v) insulin‐transferrin‐selenium (ITS; Sigma), 1.25 mg/ml bovine serum albumin (BSA; Invitrogen), 0.1 μm dexamethasone, 50 μg/ml ascorbate‐2‐phosphate, 40 μg/ml L‐proline (Sigma), 100 μg/ml sodium pyruvate (Sigma), 10 ng/ml transforming growth factor‐β1 (TGF‐β1; PeproTech, Rocky Hill, NJ, USA), 100 U/ml penicillin and 100 μg/ml streptomycin and medium was changed every 3 days. As control, cell pellets were incubated in growth medium alone.
For histochemical analyses, cells were rinsed twice in PBS, fixed overnight in PBS‐buffered 4% paraformaldehyde, dehydrated through a series of ethanols, infiltrated with isoamyl acetate and embedded in paraffin wax. Seven‐micrometer sections were cut through the centre of specimens and these were stained with alcian blue (Sigma) to demonstrate extracellular matrix glycosaminoglycans.
Proliferative potential of UC‐MSCs and BM‐MSCs
At each passage, cells were harvested using trypsin and counted after staining with trypan blue. Mean value of cell counts was calculated and mean population doubling was obtained for each passage according to the formula:
where N 0 is the initial cell number and N t harvested cell number.
Infection of UC‐MSCs and BM‐MSCs with adenovirus containing SF‐1
Full‐length SF‐1 cDNA was synthesized by reverse transcriptase‐polymerase chain reaction (RT‐PCR) and cloned into pCR‐Blunt II‐TOPO vector (Invitrogen). The entire SF‐1 cDNA sequence was verified by DNA sequencing and recombinant adenovirus vector, derived from the human type 5 adenovirus, was achieved using a commercially available adenovirus expression vector kit (TakaraBio Ltd, Shiga, Japan), containing SF‐1 cDNA, was prepared as previously described 24. UC‐MSCs and BM‐MSCs were cultured overnight at 5 × 105 cells/well density in a six‐well plates. Cells were infected with adenovirus containing SF‐1 at multiplicity of infection (MOI) of 200, and were cultured in steroidogenic differentiation medium containing DMEM‐F12 supplemented with 10% (v/v) FBS, 500 μm bdcAMP, 100 U/ml penicillin and 100 μg/ml streptomycin. Medium was changed every 2 days.
Real‐time RT‐PCR
Total RNA was isolated using RNeasy mini kit (Qiagen, GmbH, Hilden, Germany) following manufacturer's instructions, and treated with DNase I (Qiagen). Complementary DNA synthesis was performed with 1 μg total RNA using PrimeScript RTase (TaKaRa, Shiga, Japan). Real‐time RT‐PCR was conducted as previously described 3, PCR conditions including 95 °C for 15 s, 60 °C for 15 s and 72 °C for 30 s for 40 cycles using ABI 7300 Real‐Time PCR System (Applied Biosystems, Foster City, CA, USA). Expression level of β‐actin gene was used as internal control. PCR primers and probes were as follows:
P450scc:
Forward primer: 5′‐GCGGGCTCCGGAAATTACTC‐3′
Reverse primer: 5′‐CTGGTAGATGGCATCAATGAATCG‐3′
Probe: 5′‐(FAM) AGTGATGACCTGTTCCGCTTTGCCT (Eclipse)‐3′
3β‐HSD:
Forward primer: 5′‐AAGCTAGTGTGCCAGTCTTCATC‐3′
Reverse primer: 5′‐CGTTTTTCAGATTCCACCCGTTAG‐3′
Probe: 5′‐(FAM) CGCCAGTACAGCCTTCTCAGCAAGC (Eclipse)‐3′
17β‐HSD type 3 (17β‐hydroxysteroid dehydrogenase type 3):
Forward primer: 5′‐TTAGTCAACAATGTCGGAATGCTTC‐3′
Reverse primer: 5′‐ACTACGGAGGTGATGTTACAATGG‐3′
Probe: 5′‐(FAM) CCCAAGCCATTTCCTGAACGCACCG (Eclipse)‐3′
LH‐R:
Forward primer: 5′‐CAATGTGAAAGCACAGTAAGGAAAG‐3′
Reverse primer: 5′‐GGTGTCTTGGGTAAGCAGAAAC‐3′
Probe: 5′‐(FAM) CCAGCCACTCAGTTCACTCTCAGCA (Eclipse)‐3′
ACTH‐R (Adrenocorticotropic hormone receptor):
Forward primer: 5′‐CCGTGAAGTCAAGTCCAAGTAAC‐3′
Reverse primer: 5′‐CAAAACCACACGAGGACAGTC‐3′
Probe: 5′‐(FAM) CCGCCTTAACCACAAGCAGGAGAA (Eclipse)‐3′
P450c21:
Forward primer: 5′‐GCCAGTGGAGGGACATGATG‐3′
Reverse primer: 5′‐CTTCCAGGAGCTGTCCAGAG‐3′
Probe: 5′‐(FAM) CCTCTTCCATGCTCGGCTGCG (Eclipse)‐3′
CYP17:
Forward primer: 5′‐CATGCTGGACACACTGATGCAA‐3′
Reverse primer: 5′‐CGTAGAGCTTCTTCTTCACCTGA‐3′
Probe: 5′‐(FAM) AGATAATGGCAATGCTGGCCCAGAT (Eclipse)‐3′
Measurements of steroid hormones
Measurement of steroid hormones including testosterone and cortisol secreted from differentiated MSCs was performed using ELISA kits, according to manufacturer's instruction (Diagnostics Biochem Canada Inc, London, Ontario, Canada).
Analysis of cell viability
Cell viability was examined using cell counting kit‐8 (CCK‐8) (Dojindo, Kumamoto, Japan) as previously described 4. Cells were incubated at 37 °C for 30 min in 450 μl DMEM‐F12 medium (Invitrogen) and 50 μl CCK‐8 solution. Optical density was measured at 450 nm using a plate spectrophotometer.
Statistical analysis
Data are expressed as means ± SEM. Statistical comparisons were performed using Student's t‐test. P‐values <0.05 were considered statistically significant.
Results
Examination of typical MSC marker expression by flow cytometry
MSCs were isolated separately from umbilical cord Wharton's jelly and bone marrow. As expected, both UC‐MSCs and BM‐MSCs adhered to plastic surfaces and had typical spindle‐shaped appearance (data not shown).
Flow cytometric analysis indicated that both UC‐MSCs and BM‐MSCs were positive for CD105, CD90 and CD73, which are considered to be typical MSC positive markers (Fig. 1). Cells were negative for CD45, CD34, CD14, CD19 and HLA‐DR, which are typical markers of non‐MSCs (Fig. 1).
Figure 1.
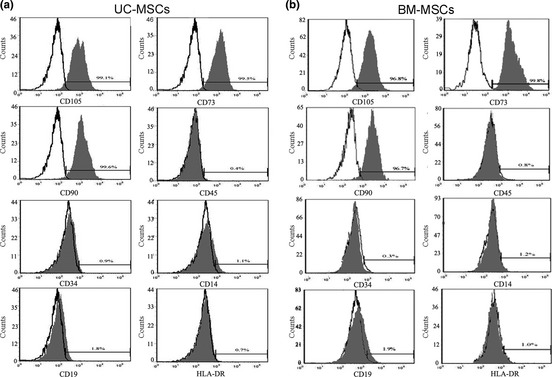
Analysis of MSC marker expression by flow cytometry. UC‐MSCs (a) and BM‐MSCs (b) were examined for expression of MSC markers. Open area represents antibody isotype control for background fluorescence, and shaded area indicates signal from antibodies to human MSC markers. Cells were stained with phycoerythrin (PE) or isothiocyanate (FITC)‐conjugated antibodies to indicated MSC markers: CD105‐PE, CD73‐PE, CD90‐PE, CD45‐PE, CD34‐FITC, CD14‐FITC, CD19‐PE and HLA‐DR‐FITC.
Examination of multipotency of UC‐MSCs and BM‐MSCs
To confirm multipotential differentiation under specific induction conditions, MSCs were also examined for adipogenic, osteogenic and chondrogenic differentiation. Both UC‐MSCs and BM‐MSCs differentiated into adipocytes after being cultured in adipogenic differentiation medium, as indicated by accumulation of neutral lipid droplets following oil red O staining (Fig. 2a2,b2); no neutral lipid droplet staining occurred when cells were cultured in growth medium only, as negative control (Fig. 2a1,b1). Both UC‐MSCs and BM‐MSCs were tested for osteogenic differentiation potential and were shown to be positive with von Kossa and ALP staining after culture in osteogenic differentiation medium, indicating that they could be induced to differentiate into osteoblasts (Fig. 2a4,a6,b4,b6). Cells were negative for von Kossa and ALP when cultured in growth medium alone, as negative control (Fig. 2a3,a5,b3,b5). Both UC‐MSCs and BM‐MSCs were also positive with alcian blue staining after culture in chondrogenic differentiation medium, indicating that they could also differentiate into chondrocytes (Fig. 2a8,b8); they were negative with alcian blue staining after culture in growth medium alone, as control (Fig. 2a7,b7). These results demonstrated that the UC‐MSCs and BM‐MSCs used in this study had typical MSC multipotential characteristics.
Figure 2.
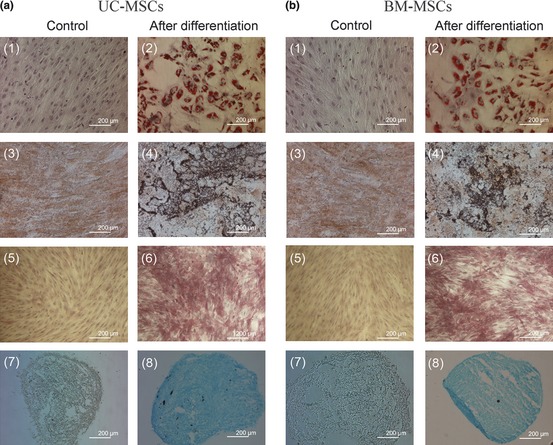
Adipogenic, osteogenic and chondrogenic differentiation of human UC ‐ MSC s and BM ‐ MSC s. UC‐MSCs (a) and BM‐MSCs (b) were examined for their multipotency. Cells were cultured in different differentiation medium for 21 days or in growth medium alone as control. Adipogenic differentiation was detected by formation of neutral lipid droplets stained with oil red O 1, 2. Osteogenic differentiation was examined using von Kossa staining, for deposition of mineralized matrix 3, 4 and ALP staining for alkaline phosphatase activity 5, 6. Chondrogenic differentiation was analysed using alcian blue staining for extracellular matrix glycosaminoglycans 7, 8.
Proliferative potential of UC‐MSCs and BM‐MSCs
UC‐MSCs and BM‐MSCs were compared for their proliferative potentials during their passages. Cells were passaged when they reached confluence and counted; numbers of UC‐MSCs increased until passage 12 and decreased over later passages. Numbers of BM‐MSCs increased up to passage 13 and decreased afterwards (Fig. 3). UC‐MSCs displayed higher cumulative population doublings with a peak of 28 at passage 12 compared to BM‐MSCs’ peak of 22 at passage 13 (Fig. 3). This indicates that UC‐MSCs had significantly higher proliferative potential than BM‐MSCs.
Figure 3.
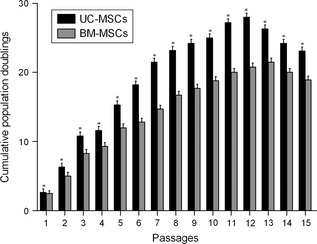
Analysis of proliferation potential of UC ‐ MSC s and BM ‐ MSC s. Cells were cultured in growth medium for 15 passages and were then harvested upon reaching confluence, stained with trypan blue and counted. Cumulative population doublings (CPD) were calculated based on cell number in each passage. Values are means ± SD (n = 3). *P < 0.01 relative to BM‐MSCs for each passage.
Morphological changes in differentiated UC‐MSCs and BM‐MSCs
UC‐MSCs and BM‐MSCs were infected with adenovirus containing SF‐1 cDNA and cultured in the presence of cAMP. Cells underwent morphological modification and changed from long fibroblast‐like cells to round or irregular polygonal shapes after being infected with adenovirus containing SF‐1, for 7 days (Fig. 4).
Figure 4.
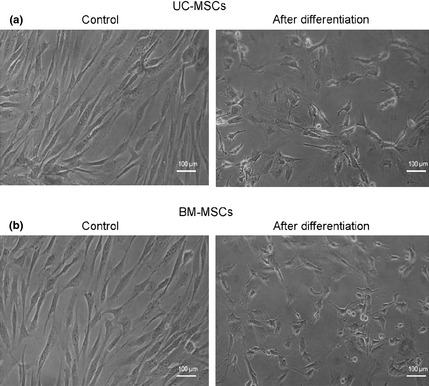
Morphological changes in UC‐MSCs and BM‐MSCs after steroidogenic differentiation. UC‐MSCs (a) and BM‐MSCs (b) were induced to become steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA, and cultured for 7 days in the presence of cAMP. Control cultures were in growth medium alone.
Detection of steroidogenic gene expression in differentiated UC‐MSCs and BM‐MSCs
UC‐MSCs and BM‐MSCs were induced to differentiate into steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA, and cultured in the presence of cAMP. Real‐time RT‐PCR was performed to detect mRNA expression of sterodogenic enzymes. Both UC‐MSCs and BM‐MSCs, following infection by adenovirus containing SF‐1 cDNA, expressed sterodogenic genes, including those for P450scc, 3β‐HSD, LH‐R, 17β‐HSD type 3, ACTH‐R, P450c21 and CYP17 (Fig. 5). When UC‐MSCs and BM‐MSCs were cultured in growth medium alone, as control, these genes were not expressed (Fig. 5). UC‐MSCs infected with adenovirus containing SF‐1 cDNA expressed significantly higher levels of all sterodogenic genes tested, over infected BM‐MSCs (Fig. 5).
Figure 5.
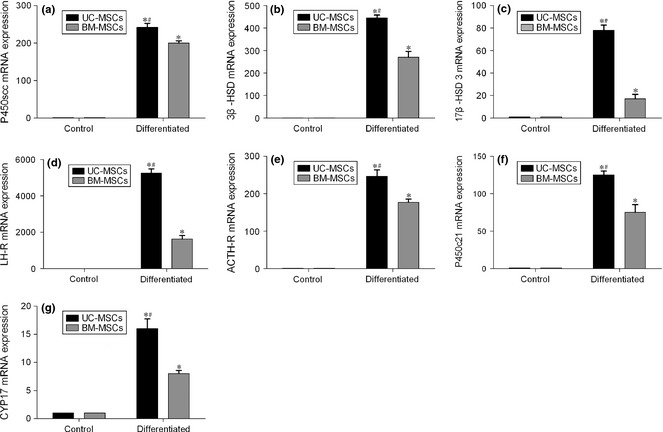
Analysis of m RNA expression of steroidogenic genes by real‐time RT ‐ PCR . UC‐MSCs and BM‐MSCs were induced to differentiate into steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA, and cultured for 7 days in the presence of cAMP. Control cultures were incubated in growth medium alone. Relative mRNA expression levels were calibrated to GAPDH and expression levels of differentiated cells are calculated relative to their respective controls. Values are means ± SD (n = 3). *P < 0.01 relative to respective controls. #P < 0.01 relative to differentiated BM‐MSCs.
Detection of viability of differentiated UC‐MSCs and BM‐MSCs
Cell viability was examined after UC‐MSCs and BM‐MSCs were infected with adenovirus containing SF‐1 cDNA and cultured in presence of cAMP for 7 days and for 14 days. Differentiated UC‐MSCs had significantly higher viability than similarly differentiated BM‐MSCs (Fig. 6). In addition, viability decreased after cells were cultured for 14 days, compared to 7 days (Fig. 6). Higher viability of differentiated UC‐MSCs could explain at least in part their higher expression of sterodogenic enzyme genes and higher secretion of sterodogenic hormones compared to the differentiated BM‐MSCs.
Figure 6.
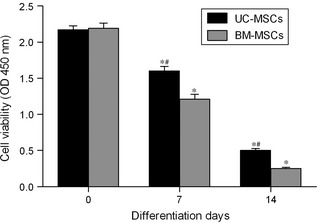
Analysis of UC ‐ MSC and BM ‐ MSC proliferation by CCK ‐8 assay. UC‐MSCs and BM‐MSCs were induced to differentiate into steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA, and cultured for 7 and 14 days in the presence of cAMP. Control cultures were incubated growth medium alone (day 0). Values are means ± SD (n = 3). *P < 0.01 relative to respective controls. #P < 0.01 relative to differentiated BM‐MSCs.
Detection of steroidogenic hormones in differentiated UC‐MSCs and BM‐MSCs
After UC‐MSCs and BM‐MSCs were infected with adenovirus containing SF‐1 cDNA and cultured in the presence of cAMP, they secreted testosterone and cortisol (Fig. 7). These two hormones were not detected in cells cultured in growth medium alone, as control. Consistent with the results of mRNA expression of steroidogenic genes (Fig. 5), infected UC‐MSCs expressed significantly higher levels of the two hormones than infected BM‐MSCs (Fig. 7).
Figure 7.
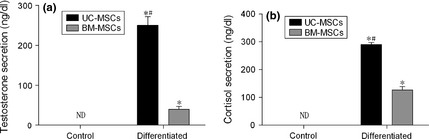
Detection of steroid secretion. Secretion of testosterone (a) and cortisol (b) in medium were detected by ELISA testing after UC‐MSCs and BM‐MSCs had been induced to differentiate into steroidogenic cells after being infected with adenovirus containing SF‐1 cDNA and cultured for 7 days, in the presence of cAMP. Control cultures were incubated in growth medium alone. Values are means ± SD (n = 3). *P < 0.01 relative to respective controls. #P < 0.01 relative to differentiated BM‐MSCs. ND, not detectable.
Discussion
This study has shown that MSCs isolated from both human bone marrow and human umbilical cord initially exhibited typical fibroblast‐like morphology and were at least 96.8% positive for MSC‐specific markers CD105, CD73 and CD90; they were negative for haematopoietic‐specific markers CD45 and CD34, for B cell‐specific markers CD14 and CD19 and for HLA‐DR, which are not expressed by non‐stimulated MSC (Fig. 1). Both UC‐MSCs and BM‐MSCs could be induced to differentiate into adipocytes, osteoblasts and chondroblasts (Fig. 2). These data clearly indicate that UC‐MSCs and BM‐MSCs used in this study had typical MSC characteristics according to criteria described by the International Society for Cellular Therapy 29.
SF‐1 is a master transcription factor in steroidogenic cell development 22. Previous research has shown that stem cells can be differentiated to steroidogenic cells using SF‐1 and cAMP. One study examined both murine and human BM‐MSC 25 and a further one also examined human BM‐MSCs 30; yet another study checked human BM‐MSCs and embryonic stem cells 26. All of these investigations clearly demonstrated that after MSCs were transfected with SF‐1 gene, cAMP treatment induced various steroidogenic enzyme expression and steroid hormone production; no cAMP treatment resulted in either no or low levels of steroidogenic enzyme expression and steroid hormone production. Molecular mechanisms underlying induction by cAMP are not clear and require further study.
Recent work has also examined steroidogenic differentiation of human umbilical cord blood‐derived MSCs (UCB‐MSCs) 31. In UCB‐MSCs, SF‐1 transfection and cAMP treatment induced expression of some steroidogenic genes and progesterone production, and other steroid hormones, such as testosterone and cortisol, were detectable at low levels. Secretion patterns of steroid hormones from the differentiated UCB‐MSCs were quite similar to those from granulosa‐luteal cells. These authors concluded that even though UCB‐MSCs could be differentiated into steroidogenic lineage by SF‐1 and cAMP, their steroidogenic properties were markedly different from BM‐MSCs. In addition, peroxisome proliferator‐activated receptor‐γ coactivator‐1α (PGC‐1α) was specifically expressed in both UCB‐MSCs and ovarian granulosa cells and PGC‐1α was involved in progesterone production in ovarian granulosa cells by enhancing SF‐1 transcriptional activity.
Here, both UC‐MSCs and BM‐MSCs exhibited morphological changes, from fibroblast‐like cells to round or irregular polygonal shapes, after being infected with adenovirus containing SF‐1, and cultured in the presence of cAMP for 7 days (Fig. 4). Real‐time RT‐PCR analysis showed that differentiated cells expressed multiple steroidogenic genes, including those for P450scc, 3β‐HSD, 17β‐HSD type 3, LH‐R, ACTH‐R, P450c21 and CYP17 (Fig. 5); they also secreted steroidogenic hormones, including testosterone and cortisol (Fig. 7). These results demonstrate that both UC‐MSCs and BM‐MSCs had the potential to differentiate into steroidogenic cells. UC‐MSCs had significantly higher proliferation than BM‐MSCs during the 15 passages tested (Fig. 3). Differentiated UC‐MSCs also had significantly higher expression of all steroidogenic mRNAs tested for, than differentiated BM‐MSCs (Fig. 5). In addition, differentiated UC‐MSCs secreted significantly more steroidogenic hormones including testosterone and cortisol compared to differentiated BM‐MSCs (Fig. 7). Furthermore, UC‐MSCs had significantly higher viability than BM‐MSCs after being infected with adenovirus containing SF‐1, for 7 and 14 days (Fig. 6). These results demonstrate that UC‐MSCs have higher potential for steroidogenic differentiation.
Umbilical cord tissue is generally discarded after birth and collection of umbilical cords does not require any invasive procedures, in contrast to isolation of bone marrow, and does not cause ethical concerns, the limitation for use of human ES cells. UC‐MSCs have lower immunogenicity than BM‐MSCs, have immunosuppressive functions and can inhibit proliferation of T cells 5, 8. UC‐MSCs have not been rejected over quite long time periods after transplantation into different species 6, 7. In addition, UC‐MSCs are multipotental and can be induced to differentiate into many types of cells 3, 4, 5. Thus, UC‐MSCs could be considered to be an ideal cell source for cell therapy compared to both BM‐MSCs and ES cells.
Luteinizing hormone (LH) is synthesized by the anterior pituitary gland and plays a key role in inducing differentiation of immature Leydig cells into mature Leydig cells 32, 33. LH is also very important for maintaining levels of proliferation of mature Leydig cells and is required to maintain physiological activity of Leydig cells during late foetal development 34, 35. LH‐R belongs to the gonadotropins and is expressed on Leydig cell membranes; binding of LH to LH‐R results in increase of testosterone synthesis by Leydig cells 36, 37. P450scc and 3β‐HSD are essential for steroid synthesis in adrenal gland and gonads 38 and type 3 17β‐HSD is mostly expressed in testis and is strongly associated with testosterone synthesis 38. In this study, UC‐MSCs showed a higher expression of steroidogenic genes, including those coding P450scc, 3β‐HSD, 17β‐HSD type 3, LH‐R, ACTH‐R, P450C21 and CYP17 after being infected with adenovirus containing SF‐1, and treated with cAMP; also, they produced more testosterone and cortisol, compared to BM‐MSCs (Figs 5, 7).
Acknowledgements
This work was supported by National Natural Science Foundation of China (grant no. 30870650 and grant no. 31171304 to Xing Wei). We thank Lizhong Liu for construction of adenoviral vectors and Jieli Pan for chondrocyte differentiation and cell proliferation analysis.
References
- 1. Bianco P, Robey PG, Simmons PJ (2008) Mesenchymal stem cells: revisiting history, concepts, and assays. Cell Stem Cell 2, 313–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Wei X, Shen CY (2011) Transcriptional regulation of Oct4 in human bone marrow mesenchymal stem cells. Stem Cells Dev. 20, 441–449. [DOI] [PubMed] [Google Scholar]
- 3. Zhang YN, Lie PC, Wei X (2009) Differentiation of mesenchymal stromal cells derived from umbilical cord Wharton's jelly into hepatocyte‐like cells. Cytotherapy 11, 548–558. [DOI] [PubMed] [Google Scholar]
- 4. Ji GZ, Wei X, Chen GQ (2009) Growth of human umbilical cord Wharton's jelly‐derived mesenchymal stem cells on terpolyester poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate‐co‐3‐hydroxyhexanoate). J. Biomater. Sci. Polymer Edn. 20, 325–339. [DOI] [PubMed] [Google Scholar]
- 5. Zhao Q, Ren H, Li X, Chen Z, Zhang X, Gong W et al (2009) Differentiation of human umbilical cord mesenchymal stromal cells into low immunogenic hepatocyte‐like cells. Cytotherapy 11, 414–426. [DOI] [PubMed] [Google Scholar]
- 6. Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H et al (2010) Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 62, 2467–2475. [DOI] [PubMed] [Google Scholar]
- 7. Medicetty S, Bledsoe AR, Fahrenholtz CB, Troyer D, Weiss ML (2004) Transplantation of pig stem cells into rat brain: proliferation during the first 8 weeks. Exp. Neurol. 190, 32–41. [DOI] [PubMed] [Google Scholar]
- 8. Tipnis S, Viswanathan C, Majumdar AS (2010) Immunosuppressive properties of human umbilical cord‐derived mesenchymal stem cells: role of B7‐H1 and IDO. Immunol. Cell Biol. 88, 795–806. [DOI] [PubMed] [Google Scholar]
- 9. Vermeulen A (2003) Diagnosis of partial androgen deficiency in the aging male. Ann. Endocrinol. (Paris) 4, 109–114. [PubMed] [Google Scholar]
- 10. Amiaz R, Seidman SN (2008) Testosterone and depression in men. Curr. Opin. Endocrinol. Diabetes Obes. 15, 278–283. [DOI] [PubMed] [Google Scholar]
- 11. Kalyani RR, Dobs AS (2007) Androgen deficiency, diabetes, and the metabolic syndrome in men. Curr. Opin. Endocrinol. Diabetes Obes. 14, 226–234. [DOI] [PubMed] [Google Scholar]
- 12. Vermeulen A (2001) Androgen replacement therapy in the aging male – a critical evaluation. J. Clin. Endocrinol. Metab. 86, 2380–2390. [DOI] [PubMed] [Google Scholar]
- 13. Lunenfeld B, Nieschlag E (2007) Testosterone therapy in the aging male. Aging Male 10, 139–153. [DOI] [PubMed] [Google Scholar]
- 14. Boyanov MA, Boneva Z, Christov VG (2003) Testosterone supplementation in men with type 2 diabetes, visceral obesity and partial androgen deficiency. Aging Male 6, 1–7. [PubMed] [Google Scholar]
- 15. Finas D, Bals‐Pratsch M, Sandmann J, Eichenauer R, Jocham D, Diedrich K et al (2006) Quality of life in elderly men with androgen deficiency. Andrologia 38, 48–53. [DOI] [PubMed] [Google Scholar]
- 16. Chen H, Ge RS, Zirkin BR (2009) Leydig cells: from stem cells to aging. Mol. Cell. Endocrinol. 306, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zirkin BR, Ewing LL (1987) Leydig cell differentiation during maturation of the rat testis: a stereological study of cell number and ultrastructure. Anat. Rec. 219, 157–163. [DOI] [PubMed] [Google Scholar]
- 18. Shan LX, Phillips DM, Bardin CW, Hardy MP (1993) Differential regulation of steroidogenic enzymes during differentiation optimizes testosterone production by adult rat Leydig cells. Endocrinology 133, 2277–2283. [DOI] [PubMed] [Google Scholar]
- 19. Ge RS, Dong Q, Sottas CM, Papadopoulos V, Zirkin BR, Hardy MP (2006) In search of rat stem Leydig cells: identification, isolation, and lineage‐specific development. Proc. Natl. Acad. Sci. USA 103, 2719–2724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Su Y, Rose JC (2008) The impact of ACTH receptor knockdown on fetal and adult ovine adrenocortical cell function. Reprod. Sci. 15, 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Arase M, Waterman MR, Kagawa N (2006) Purification and characterization of bovine steroid 21‐hydroxylase (P450c21) efficiently expressed in Escherichia coli . Biochem. Biophys. Res. Commun. 344, 400–405. [DOI] [PubMed] [Google Scholar]
- 22. Lala DS, Rice DA, Parker KL (1992) Steroidogenic factor I, a key regulator of steroidogenic enzyme expression, is the mouse homolog of fushi tarazu‐factor I. Mol. Endocrinol. 6, 1249–1258. [DOI] [PubMed] [Google Scholar]
- 23. Sadovsky Y (1995) Mice deficient in the orphan receptor steroidogenic factor 1 lack adrenal glands and gonads but express P450 sidechain‐cleavage enzyme in the placenta and have normal embryonic serum levels of corticosteroids. Proc. Natl. Acad. Sci. USA 92, 10939–10943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tanaka T, Gondo S, Okabe T, Ohe K, Shirohzu H, Morinaga H et al (2007) Steroidogenic factor 1/adrenal 4 binding protein transforms human bone marrow mesenchymal cells into steroidogenic cells. J. Mol. Endocrinol. 39, 343–350. [DOI] [PubMed] [Google Scholar]
- 25. Yazawa T, Mizutani T, Yamada K, Kawata H, Sekiguchi T, Yoshino M et al (2006) Differentiation of adult stem cells derived from bone marrow stroma into Leydig or adrenocortical cells. Endocrinology 147, 4104–4111. [DOI] [PubMed] [Google Scholar]
- 26. Yazawa T, Kawabe S, Inaoka Y, Okada R, Mizutani T, Imamichi Y et al (2011) Differentiation of mesenchymal stem cells and embryonic stem cells into steroidogenic cells using steroidogenic factor‐1 and liver receptor homolog‐1. Mol. Cell. Endocrinol. 336, 127–132. [DOI] [PubMed] [Google Scholar]
- 27. Hu YJ, Wei X, Zhao W, Liu YS, Chen GQ (2009) Biocompatibility of poly(3‐hydroxybutyrate‐co‐3‐hydroxyvalerate‐co‐3‐hydroxyhexanoate) with bone marrow mesenchymal stem cells. Acta Biomater. 5, 1115–1125. [DOI] [PubMed] [Google Scholar]
- 28. Wei X, Hu YJ, Xie WP, Lin RL, Chen GQ (2009) Influence of poly(3‐hydroxybutyrate‐co‐4‐hydroxybutyrate‐co‐3‐hydroxyhexanoate) on growth and osteogenic differentiation of human bone marrow‐derived mesenchymal stem cells. J. Biomed. Mater. Res. A 90, 894–905. [DOI] [PubMed] [Google Scholar]
- 29. Dominici M, Le Blanc K, Mueller I, Slaper‐Cortenbach I, Marini F, Krause D et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- 30. Yazawa T, Inanoka Y, Mizutani T, Kuribayashi M, Umezawa A, Miyamoto K (2009) Liver receptor homolog‐1 regulates the transcription of steroidogenic enzymes and induces the differentiation of mesenchymal stem cells into steroidogenic cells. Endocrinology 150, 3885–3893. [DOI] [PubMed] [Google Scholar]
- 31. Yazawa T, Inaoka Y, Okada R, Mizutani T, Yamazaki Y, Usami Y et al (2010) PPAR‐γ coactivator‐1α regulates progesterone production in ovarian granulosa cells with SF‐1 and LRH‐1. Mol. Endocrinol. 24, 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ge RS, Shan LX, Hardy MP (1996) Pubertal development of Leydig cells In: Payne AH, Hardy MP, Russel LD, ed. The Leydig Cell, pp. 160–173. Vienna, IL: Cache River Press. [Google Scholar]
- 33. Habert R, Lejeune H, Saez JM (2001) Origin, differentiation and regulation of fetal and adult Leydig cells. Mol. Cell. Endocrinol. 179, 47–74. [DOI] [PubMed] [Google Scholar]
- 34. Benton L, Shan LX, Hardy MP (1995) Differentiation of adult Leydig cells. J. Steroid Biochem. Mol. Biol. 53, 61–68. [DOI] [PubMed] [Google Scholar]
- 35. Bortolussi M, Zanchetta R, Belvedere P, Colombo L (1990) Sertoli and Leydig cell numbers and gonadotrophin receptors in rat testis from birth to puberty. Cell Tissue Res. 260, 185–191. [DOI] [PubMed] [Google Scholar]
- 36. Ascohi MC (1985) Regulation of luteinizing hormone receptors and actions In: Ascoli M, ed. Luteinizing Hormone Action and Receptors, pp, 199–219. Boca Raton, FL: CRC Press. [Google Scholar]
- 37. Hussein MO, Zipf WB (1987) Characteristics of prolactin‐modulated LH induction of LH/hCG receptors. Transient inhibition of receptor induction following prolactin exposure. J. Androl. 8, 388–392. [DOI] [PubMed] [Google Scholar]
- 38. Yanase T, Gondo S, Okabe T, Tanaka T, Shirohzu H, Fan W et al (2006) Differentiation and regeneration of adrenal tissues: an initial step toward regeneration therapy for steroid insufficiency. Endocr. J. 53, 449–459. [DOI] [PubMed] [Google Scholar]


