Abstract
Abstract. Although progress has been made with respect to the growth and transcription factors implicated in pancreatic development, many questions remain unsolved. It has been established that during embryonic life, both endocrine and acinar cells are derived from pancreatic epithelial precursor cells. Growth factors control the proliferation of precursor cells and their ability to differentiate into mature cells, both in pre‐natal and in early post‐natal life. Pancreatic development during the early post‐natal period is an area of great interest for many scientists. In this study we have examined the structure characteristics, functional and proliferative activity of control and diabetic hamster pancreatic ductal, exocrine and β cells, following treatment with FGFs 1, 2 and 7 in vitro. Light and electron microscopic studies indicated active synthetic processes in these cells under the influence of the investigated FGFs. In our experimental model of diabetes, the labelling index of the cells was significantly higher than in corresponding control groups of hamsters. We established that FGF2 at a concentration of 10 ng/l was responsible for the most prominent effect on ductal cells and β cells in the diabetic groups. FGF1 at a concentration of 10 ng/l displayed the highest stimulatory effect on exocrine cells in the diabetic groups at post‐natal day 10. Taken together these data strongly suggest that FGF1 and FGF2 induce proliferation of pancreatic epithelial cells during the early post‐natal period whereas FGF7 is not strictly specific for pancreatic cell proliferation.
INTRODUCTION
Epithelial mesenchymal interactions are important for both morphogenesis and differentiation of the pancreas. Information concerning the role of mesenchymal factors in pancreatic development is still controversial and incomplete. Several investigators have reported that cells residing in the duodenal anlage cannot differentiate into mature ductular, endocrine and acinar pancreatic structures in the absence of mesenchymal factors (Golosow & Grobstein 1962; Wessels & Cohen 1967; Fell & Grobstein 1968; Rao et al. 1990; Dudek et al. 1991; Gu & Sarvetnick 1993). Other studies (Gittes et al. 1996) have shown that epithelial cells that experience no mesenchymal signalling mainly form clusters of mature islets.
There are two mechanisms by which pancreatic cells develop: differentiation of ductal precursor cells (neogenesis) and proliferation of pre‐existing differentiated cells (Bonner‐Weir & Smith 1994; Finegood et al. 1995; Vinik et al. 1996; Arany & Hill 2000). The process of neogenesis in mammals generally takes place during foetal and neonatal life. Differentiation of beta cells from undifferentiated precursors during early embryogenesis is the area which has been studied most frequently. It has been established that when embryonic (E)12.5 rat pancreatic rudiments are cultured in vitro in the absence of mesenchyme, the majority of the epithelial cells differentiate into endocrine β cells. Signals from the mesenchyme activate epithelial cell proliferation but concomitantly suppress the differentiation of pancreatic epithelium into endocrine cells (Miralles et al. 1999). Epidermal growth factor (EGF) acts in a stimulatory capacity on the embryonic pancreatic epithelium, and in culture conditions, EGF initiates epithelial precursor cell proliferation, whereas endocrine cell differentiation is repressed (Cras‐Meneur et al. 2001). In the basement membrane‐rich gel, E11.5 mouse pancreatic epithelium cultured without mesenchyme forms ductal structures. When pancreatic epithelial cells are grown beneath the renal capsule of adult mice without mesenchymal interaction, clusters of mature islet cells form (Gittes 1996). In the absence of an external stimulus, rat β cells exhibit low mitotic activity after weaning, which remains into adulthood (Garofano et al. 2000). To investigate mechanisms responsible for abnormal β cell development, resulting in impaired glucose homeostasis, neonatal experimental models of diabetes are commonly used. In rats treated with streptozotocin (STZ), loss of β‐cell mass leads to diabetes, followed by the reappearance of new β cells (Portha et al. 1974; Bonner‐Weir et al. 1981; Ferrand et al. 1995).
In laboratory rodents, treatment with STZ causes β‐cell death with subsequent hyperglycaemia. After STZ‐mediated elimination of mature β cells, insulin‐containing intra‐islet cells appear but their number is low and the animals remain hyperglycaemic. This condition of the animals, however, is improved after exogenous administration of insulin, which also has a beneficial effect on β‐cell regeneration (Guz et al. 2001).
It has previously been established that in general, two types of signals regulate cell proliferation, differentiation and survival: (1) signalling by soluble regulatory molecules (hormones and growth factors) and (2) signals delivered by extracellular matrix (ECM) proteins. Among the growth factors that influence pancreatic development are members of the fibroblast growth factor (FGF) family. Evidence exists that FGFs promote pancreatic epithelial cell proliferation via FGF receptors (FGFRs). It is generally accepted that FGFs play a role in the development of many cell types and act as both autocrine and paracrine mediators of differentiation. Islet‐cell regeneration in the diabetic hamster pancreas can be induced by local growth factors (Rosenberg et al. 1996).
The aim of the present study was to examine dose‐dependent effects of FGF1, FGF2 and FGF7 at three post‐natal days (pnd 1, 5 and 10), on pancreatic ductal, exocrine and β‐cell proliferation in FGF‐treated healthy hamster groups (controls) and in FGF‐ and STZ‐treated (diabetic) hamster groups.
MATERIALS AND METHODS
Experimental diabetes in fertile adult female hamsters was induced during the first 24 h after mating by an intraperitoneal STZ injection at a dose of 65 mg/kg into the mother. Only animals from litters with blood glucose levels over 11 mmol/l were used. Each group of experimental diabetic animals consisted of 20 hamsters at pnd 1, 5 and 10. The number of healthy hamsters was 5, 10 and 7 animals, respectively. Healthy and diabetic pups were killed by cervical dislocation and the pancreata were aseptically dissected and cut into small segments. One or two pancreatic segments were cultured in each well of 96‐well sterile plastic plates (Linbro, Flow Laboratories, Irvine, UK). These pancreatic organ cultures were grown in Iscove's Modified Dulbecco's Medium (Sigma‐Aldrich, St. Louis, MO, USA), supplemented with 10% foetal bovine serum (BioWhittaker Europe, Verviers, Belgium), 100 U/ml penicillin and 100 µg/ml streptomycin. The incubation medium was supplemented with 4, 10 and 100 ng/l FGF1, FGF2 and FGF7 for treatment of equal numbers of organ cultures from diabetic and normal hamsters. All pancreatic tissue pieces were cultured at 37 °C in a humidified CO2 incubator (5% CO2/95% air). After 48 h 2 µCi [3H] dT/ml was added to the wells and pancreatic organ cultures were incubated for a further 8 h. The tissue segments were fixed in Bouin's fluid for 24 h, dehydrated in graded ethanols, paraffin‐wax embedded, sectioned and stained with haematoxylin and eosin (H&E) for light microscopic observation. Paraldehyde fuchsine staining (PAF) (Gomori 1950) was used as a specific histochemical method for detection of insulin‐synthesizing cells. Autoradiography was performed according to Larra & Droz (1970). At least six silver grains per nucleus were considered as a marker of a positive reaction.
For electron microscopy, small fragments were fixed by immersion in 0.1 m phosphate‐buffered (pH 7.4) 1% glutaraldehyde for 2 h at room temperature, post‐fixed with 0.1 m phosphate buffered 1% osmium tetroxide for 1 h at 4 °C, and were dehydrated and embedded in Durcopan, according to standard procedures. Ultrathin sections, mounted on grids, were stained with uranyl acetate and lead citrate and sections were examined in a Siemens 101 electron microscope.
Proliferation of pancreatic cells was determined by the labelling index (LI) according to the formula: LI (%) = number of labelled nuclei/number of counted nuclei × 100.
At least 500 ductal, exocrine and endocrine cells were evaluated from growth factor‐treated and ‐untreated pancreatic organ cultures, obtained from each experimental group of animals.
Student's t‐test was used for statistical analysis (statmost program).
RESULTS
Pancreatic organ cultures from control hamsters were distinguished by their well‐developed mesenchymal tissue, appearance of ductal structures (mostly at the periphery) and differentiated acinar cells. An increase in proliferative areas after treatment with FGFs was observed as a common feature. Pancreatic organ cultures from all diabetic groups had more ductal structures and more proliferating ductal cells in comparison with the respective control groups.
In growth factor‐untreated groups, endocrine cells formed small aggregates, like islets of Langerhans, either in direct contact with the surrounding exocrine cells or separated from the exocrine parenchyma by connective tissue.
In the organ cultures from control groups at pnd 5, treated with 10 ng/l FGF1, an abundance of mesenchymal tissue was observed. We also observed a process of formation of islets of Langerhans at the earliest time period (pnd 1) investigated. At the stages pnd 5 and 10, large numbers of islets were found throughout the mesenchymal tissue as well as throughout the exocrine parenchyma in these specimens (Fig. 1).
Figure 1.
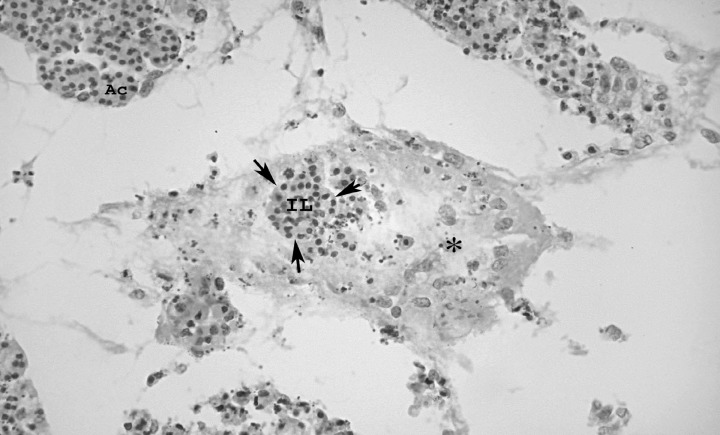
Islets of Langerhans throughout mesenchymal tissue in the pancreas of a control specimen, stage pnd 5, after treatment with 10 ng/l FGF7; IL, islet of Langerhans; Ac, acinar cells; asterisk‐mesenchymal tissue; H&E, ×25 magnification.
In pancreatic samples from diabetic hamsters we found PAF‐positive secretions in the ductal luminae and more PAF‐positive cells among ductal epithelial cells than in the corresponding groups of control animals. We also established the presence of more PAF‐positive cells close to the ducts and also scattered among the acinar cells, in the pancreata of diabetic hamsters in comparison with the control group at pnd 5 after treatment with 10 ng/l FGF2 (52 versus 31).
The electron microscopic study provided us with further information concerning the effect of FGFs on pancreatic cell morphology. In relation to active synthetic processes in pancreata from control and diabetic groups, we observed secretory β granules in different degrees of maturation. β Granules have a recognizable specific shape, typically spherical, the core electron dense and a light halo; further, granules consisted of several electron‐dense bodies. In the diabetic animal groups, a substantial quantity of rough‐surfaced lamellae of the endoplasmic reticulum plus an abundance of free polyribosomes was present, as well as markedly lengthened mitochondria. There were ‘coated’ vesicles in some of the β cells, which suggested active processes of intracellular transport (Fig. 2).
Figure 2.
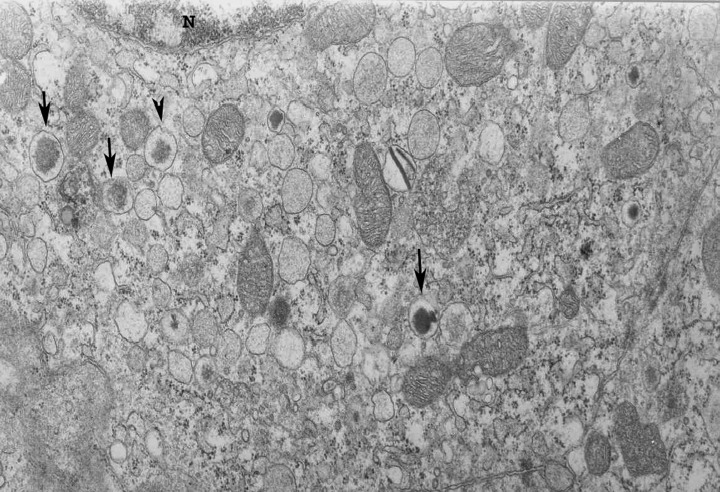
Portion of B cell in the pancreas of control specimen stage pnd 10 after treatment with 100 ng/l FGF1. N, nucleus; arrows, granules of β cell in different stages of maturation; ×30 000 magnification.
To evaluate the mitogenic effect of FGFs on the pancreatic epithelial cells, we applied the histoautoradiographic method. The histoautoradiograms revealed proliferating ductal cells at the periphery and proliferating exocrine and endocrine cells in the central part of the pancreatic tissue in samples from control hamsters. In those from the diabetic groups, more marked nuclei, mostly at the expense of proliferating ductal cells, were observed (Fig. 3).
Figure 3.
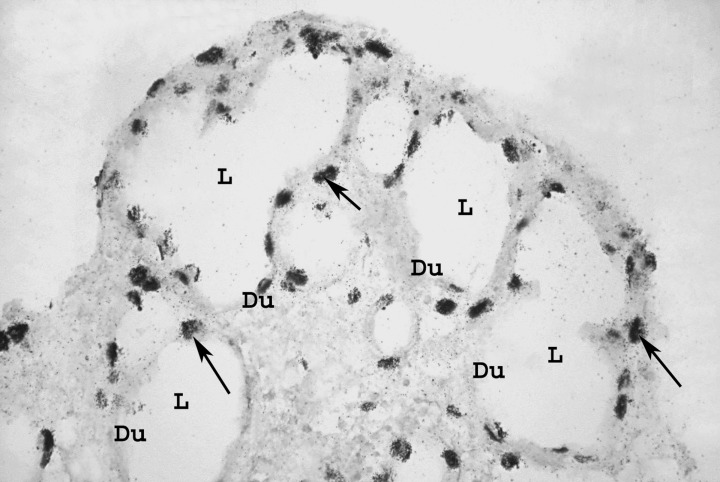
Marked nuclei in ductal epithelium of the diabetic group stage pnd 5, after treatment with 100 ng/l FGF1. Du, duct; L, ductal lumen; arrows, marked nuclei; ×100 magnification.
In FGF‐untreated diabetic organ cultures, ductal cells had the highest LI at pnd 5, compared to LI of the same cells from FGF‐untreated healthy group (4 ± 0.6 versus 2.8 ± 0.3%, P < 0.05). The LI of exocrine cells was the highest also at pnd 5 (2.5 ± 0.3 versus 1.3 ± 0.2%, P < 0.01). No statistical differences of LI of β cells within the same groups were observed (Fig. 4). In all, FGF‐stimulated organ cultures of pancreatic cell proliferation have shown a dose‐dependent effect.
Figure 4.
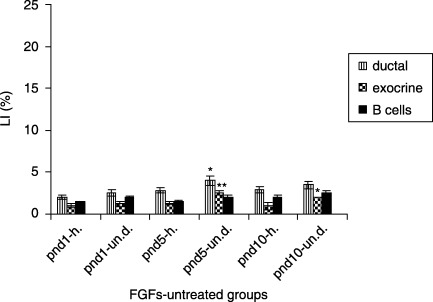
Proliferative activity of FGF‐untreated experimental groups (mean ± SE); h, FGF‐untreated healthy group; un. d, FGF‐untreated diabetic group; significant difference between FGF‐untreated healthy group *P < 0.05; **P < 0.01; ***P < 0.001.
In the 4 ng/l FGF1‐treated diabetic groups the ductal cells showed the highest LI at pnd 10, compared to the control groups (8.0 ± 0.6 versus 4.5 ± 0.6%, P < 0.01). The β cells had the highest LI at pnd 5 (3 ± 0.2 versus 1.2 ± 0.8%, P < 0.01), whereas the exocrine cells had the highest LI at pnd 1 (3.0 ± 0.4 versus 2 ± 0.3%, P < 0.05) (Fig. 5).
Figure 5.
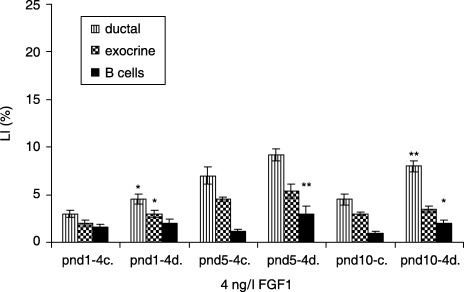
Effect of 4 ng/l FGF1 on control and diabetic groups (mean ± SE); c, control group; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
In the 10 ng/l FGF1‐treated diabetic groups, the exocrine cells had the highest LI at pnd 10 compared to LI of the control (5.5 ± 0.2 versus 4.0 ± 0.3%, P < 0.05). The β cells had the highest LI at the same age (3.3 ± 0.2 versus 2.0 ± 0.4, P < 0.01). No statistical differences of LI of the ductal pancreatic cells were observed (Fig. 6).
Figure 6.
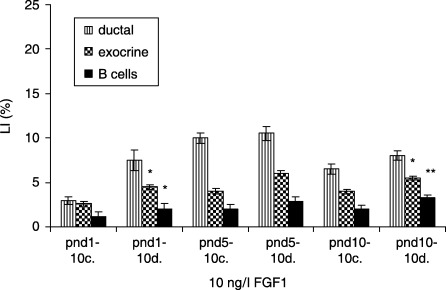
Effects of 10 ng/l FGF1 on control and diabetic groups (mean ± SE); c, control; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
In the 100 ng/l FGF1‐treated diabetic groups, ductal cells had the highest LI at pnd 5, compared to the controls (10.2 ± 0.3 versus 9.0 ± 0.4%, P < 0.01). Exocrine cells revealed the highest LI at pnd 5 (5.3 ± 0.4 versus 4.5 ± 0.6, P < 0.05), and no statistical differences were observed in β cell LI (Fig. 7). In FGF2‐treated (4 ng/l) diabetic groups, ductal cells displayed the highest LI at pnd 5, compared to controls (10.2 ± 0.3 versus 9.0 ± 0.4%, P < 0.01). LI of exocrine cells was highest at pnd 5 (5.3 ± 0.4 versus 4.5 ± 0.6%, P < 0.05), whereas β cells displayed the highest LI at pnd 10 (2.5 ± 0.4 versus 1.5 ± 0.4%P < 0.01) (Fig. 8).
Figure 7.
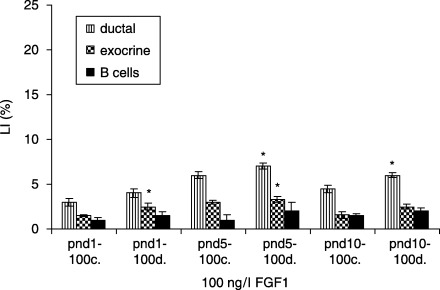
Effects of 100 ng/l FGF1 on control and diabetic groups (mean ± SE); c, control; d, diabetic groups; significant differences from control groups *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 8.
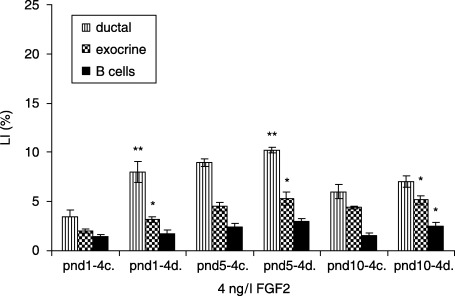
Effects of 4 ng/l FGF2 on control and diabetic groups (mean ± SE); c, control; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
In 10 ng/l FGF2‐treated diabetic groups, ductal cells (17.5 ± 0.9 versus 12.0 ± 0.4%, P < 0.001) and exocrine cells (5.4 ± 0.4 versus 3.0 ± 0.2%, P < 0.01) revealed highest LIs at pnd 5, compared to controls. β cells had the highest LIs at pnd 5 (4 ± 0.4 versus 1.3 ± 0.3%, P < 0.001) and at pnd 10 (4 ± 0.8 versus 3.0 ± 0.4%, P < 0.05) (Fig. 9). In 100 ng/l FGF2‐treated diabetic groups, ductal cells showed the highest LI at pnd 1, compared to controls (7.0 ± 0.6 versus 3.0 ± 0.5, P < 0.001), and the LI of β cells was the highest at the same point in gestation (1.8 ± 0.9 versus 1.2 ± 0.3%, P < 0.05), whereas no statistical difference in LIs of exocrine cells in the same groups was observed (Fig. 10).
Figure 9.
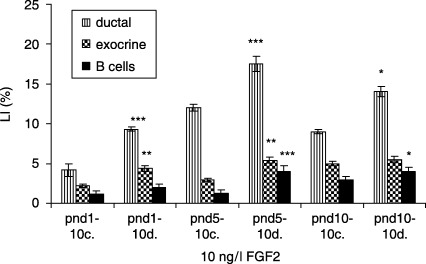
Effects of 10 ng/l FGF2 on control and diabetic groups (mean ± SE); c, control group; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 10.
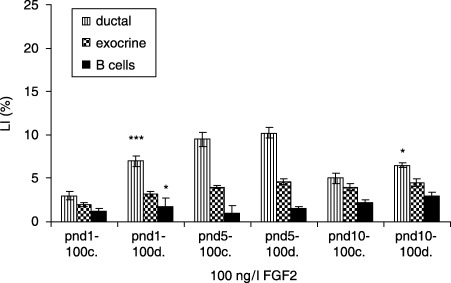
Effects of 100 ng/l FGF2 on control and diabetic groups (mean ± SE); c, control group; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
In FGF7‐treated (4 ng/l) diabetic groups, ductal cells had the highest LI at pnd 5, compared to controls (7.8 ± 0.6 versus 5.7 ± 0.5%, P < 0.001). The exocrine cells in the respective experimental categories showed the highest LI at pnd 1 (4.8 ± 0.3 versus 1.2 ± 0.2%, P < 0.001). β cells revealed the highest LI at pnd 5 (2.6 ± 0.3 versus 2.0 ± 0.3%, P < 0.05) (Fig. 11). In 10 ng/l FGF7‐treated diabetic groups, ductal cells showed the highest LI at pnd 10, compared to controls (9.8 ± 0.3 versus 7.0 ± 0.6%, P < 0.01). The exocrine cells in the same experimental groups displayed the highest LI at pnd 10 (3.0 ± 0.8 versus 2.0 ± 0.2, P < 0.05), whereas β cells had the highest LI at pnd 5 (3.0 ± 0.3 versus 2.5 ± 0.3, P < 0.05) (Fig. 12). In the diabetic groups treated with 100 ng/l FGF7, ductal cells (7.0 ± 0.3 versus 5.0 ± 0.6%, P < 0.01) as well as β cells (2.0 ± 0.2 versus 1.5 ± 0.4, P < 0.05) had the highest LI at pnd 5, compared to controls. The exocrine cells in the same experimental groups presented no statistical differences in LIs (Fig. 13).
Figure 11.
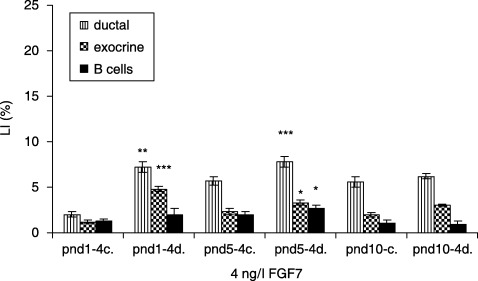
Effects of 4 ng/l FGF7 on control and diabetic groups (mean ± SE); c, control; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 12.
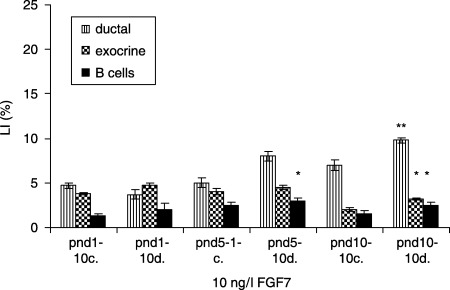
Effects of 10 ng/l FGF7 on control and diabetic groups (mean ± SE); c, control group; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
Figure 13.
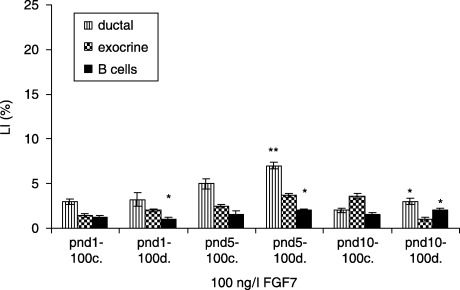
Effects of 100 ng/l FGF7 on control and diabetic groups (mean ± SE); c, control group; d, diabetic group; significant differences from control group *P < 0.05; **P < 0.01; ***P < 0.001.
DISCUSSION
The present study investigated the effects of three FGFs – FGF1, FGF2 and FGF7 – on the processes of cell proliferation and differentiation of pancreatic ductal, exocrine and β cells from control categories, and in STZ‐diabetic hamster groups. Our experimental data have demonstrated an increase in proliferative activity of the three types of studied pancreatic cells.
During embryonic and post‐natal periods, development of mature pancreatic tissue is thought to require proliferation of precursor cells followed by their differentiation into acinar and/or endocrine cells. These processes are controlled by growth factors produced by supporting mesenchymal cells (Le Bras et al. 1998; Cras‐Meneur 2001). A considerable number of studies has indicated that FGFs 1, 2, 7, 9, 10, 11 and 18 are expressed in the pancreas at varying time points and levels during adulthood (Le Bras et al. 1998; Arany & Hill 2000; Hart et al. 2000; Dichmann et al. 2003; Movassat et al. 2003). Nevertheless, little is understood of their contributions to pre‐natal and post‐natal development of the pancreas. In the present study on effects of FGF1, FGF2 and FGF7 on early pancreatic post‐natal development, of control and STZ‐diabetic hamsters, it has been found that these growth factors induce proliferation of the pancreatic ductal, exocrine and β cells. In the investigated FGF‐untreated healthy groups we established that ductal, exocrine and β cells showed lower LIs in comparison to the same pancreatic cell types in FGF‐untreated diabetic hamsters. Ductal and exocrine cells in the groups treated with STZ at age pnd 5 displayed the highest values of LI, which were significantly different. We explain these results by observing that STZ causes fragmentation of the pancretic β‐cell DNA and subsequent necrosis of the β cells. This leads to homeostatic compensation of the remaining pancreatic tissue by increasing proliferative activity of β cells.
It is well known that β cells have poor regenerative capacity in adults and that STZ induces β cell destruction and irreversible diabetes (Yamamoto et al. 2004). In the early post‐natal period, β cells have a good ability to regenerate (Portha et al. 1974; Bonner‐Weir 1981; Ferrand et al. 1995). In our experimental model of diabetes, LIs of three of pancreatic cell types was higher than in corresponding groups of control hamsters. The LI of pancreatic cells was the highest at age pnd 5 and pnd 10 in the diabetic groups. At the same time, in all experimental groups (control and diabetic) treated with FGF1, FGF2 and FGF7, the LIs of ductal, exocrine and β cells were higher than in untreated tissues by the same growth factors groups. Therefore, the investigated FGFs increased pancreatic ductal, exocrine and β‐cell proliferation activity in control groups and mostly in our experimental model of diabetes. In fact, these results are in agreement with a large number of pieces of data that FGFs promote cell proliferation and differentiation of pancreatic epithelial cells both in vitro and in vivo (Hardikar et al. 2003; Movassat et al. 2003; Ye et al. 2005). Our experiments indicated that ductal cells have the highest proliferative activity out of all the experimental groups. There are many studies that provide evidence that the proliferation of pancreatic ductal cells during embryonic life and in adults leads to an increase in the number of precursor cells that could differentiate into acinar or β cells (Sarvetnick et al. 1990; Scharma et al. 1999; Plachot & Portha 2001). Furthermore, neogenesis from the ductal epithelium is feasible in the adult gland in various models of pancreatic regeneration, such as partial pancreatectomy, duct ligation, cellophane wrapping or γ‐interferon over‐expression (Rosenberg et al. 1983; Bonner‐Weir et al. 1993; Gu & Sarvetnick 1993; Wang et al. 1995). In many cases, endocrine cells have also been found in the wall of the ducts (Bendayan 1987). In vitro culture of human islets from expanded ductal tissue further suggests that β cells could be derived from ductal cells (Bonner‐Weir et al. 2000). It has been reported that BrdU retention is a property of pancreatic progenitor cells located within the islets of Langerhans (Duvillie et al. 2003). The most important finding in our study was that the ductal cells have provided the highest LI at age pnd 5 in diabetic groups after treatment with 10 ng/l FGF2. We have also established that β cells have displayed the highest LI at pnd 5 and 10 in diabetic groups after treatment with 10 ng/l FGF2. These results bring additional pieces of evidence in support of the statement that pancreatic β cells could be derived from duct cells precursors. Moreover, FGF2 had the strongest mitogenic effect on both ductal and β cells after STZ‐mediated destruction of the pancreatic β cells.
We observed a stronger effect of FGF1 in comparison with FGF7, both in control specimens and in the diabetic groups of hamster tissue, and a lesser effect in comparison with treatment by FGF2. These results correlate well with the data that FGF1 and vascular endothelial growth factor provide an angiogenic effect (Xu & Greisler 2002). We have established that FGF7 has shown the lowest mitogenic effect both in control and in diabetic experimental groups. There is information that FGF7 enhances intra‐islet ductal cell proliferation in the pancreas and leads to differentiation of islets of hepatocytes (Krakowski et al. 1999). There are data that show that FGF7 is not an obligatory growth factor for pancreatic endocrine development as gene inactivation, caused by homologous recombination, results in viable offspring without any obvious metabolic or growth defects (Guo et al. 1996). Based on investigations of these authors as well as on our results, we assume that although FGF7 can induce epithelial cell proliferation, it is not strictly specific for the pancreatic endocrine cell proliferation.
FGF1, FGF2 and FGF7 have shown a dose‐dependent effect on pancreatic epithelial cell proliferation. In our study we have established that at concentrations of 4 and 10 ng/l, these growth factors induce proliferation of more ductal, exocrine and β cells, whereas at a concentration of 100 ng/l, their effect decreased both in the control and diabetic groups. These results are in agreement with our previous experiments (Ogneva & Martinova 2002).
On the other hand, we have also established an age‐dependent loss of the proliferative potential of the pancreatic cells in diabetic as well as in the control groups. In FGF‐untreated groups we established that ductal and exocrine cells displayed significantly highest values of LI at pnd 5 after treatment with STZ. In the diabetic groups we registered the highest values of LI of ductal cells at age pnd 5 (significantly different), whereas β cells displayed equal LIs at pnd 5 and 10 after FGF2 treatment. These results are in agreement with the concept that the age‐dependent loss of proliferative potential of differentiated endocrine cells prevents the formation of terminally mature islets and explains the incomplete recovery of the islet mass (Ferrand et al. 1995) and what is more, it has been established that the steady‐state level of FGFR1 mRNA were maximum during early embryonic life, embryonal (days 14–16) and decreased later to beneath the limit of detection in the adult rat pancreas (Le Bras et al. 1998). It has also been found that multiple FGF species are widespread at pnd 12–14 when a wave of ductal cell proliferation and endocrine cell neogenesis is thought to take place (Arany & Hill 2000).
To gain a more precise understanding of the processes of proliferation and differentiation in the post‐natal development of the pancreas, we have used PAF staining, which is specific for insulin‐synthesizing cells. We observed numerous PAF‐positive cells in the ductal epithelium. PAF staining has shown a PAF‐positive secretion in the ducts as well. There are data in the literature that the epithelia grown in basement membrane‐rich gel form multiple cystic structures. These cells stain positively for the pancreatic ductal antigen (cytoceratin 7). There are also insulin‐positive cells (Gittes et al. 1996).
In connection with the mitogenic effect of investigated FGF1, FGF2 and FGF7, we have observed a well‐developed rough‐surfaced endoplasmic reticulum lamella, polyribosomes and mitochondria in the duct cells as well as in the β cells in the control and diabetic groups. Our ultrastructural analysis indicated the presence of very numerous ductal cells as well as α cells, close to the ductal cells. We have not found β cells close to ductal cells at the ultrastructural level. In STZ‐treated newborn rats, a previously unknown polymorphous endocrine cell type subpopulation (PEPS) has been identified. Ultrastructural analysis revealed the presence of PEPS close to the duct cells and inserted in an endocrine cell cluster (Ferrand et al. 1995).
In summary, we have established that there is an increase in proliferative activity of the ductal, exocrine and β cells in organ cultures of diabetic hamster pancreata, in comparison with appropriate controls. Our results provide evidence for the strong mitogenic effect of FGF2, especially on the ductal and β cells in the diabetic groups at a concentration of 10 ng/l. FGF1 at a concentration 10 ng/l showed its most stimulatory effect on the exocrine cells of the diabetic group at pnd 10. The data strongly suggest that FGF1 and FGF2 induce proliferation of pancreatic epithelial cells during the early post‐natal period, whereas FGF7 is not specific for pancreatic cell proliferation. Our results concerning higher LI values in diabetic groups after treatment with FGF2 probably indicate compensatory pancreatic formation of β cells, which supports the hypothesis of S. Bonner‐Weir et al. (2000), suggesting a possible role for ductal cells as β‐cell precursors.
REFERENCES
- Arany E, Hill DJ (2000) Ontogeny of fibroblast growth factors in the early development of the rat endocrine pancreas. Pediatr. Res. 48 (3), 389–403. [DOI] [PubMed] [Google Scholar]
- Bendayan M (1987) Presence of endocrine cells in pancreatic ducts. Pancreas 2 (4), 393–397. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S, Smith FE (1994) Islet cell growth and the growth factors involved. Trends Endocrinol. Metab. 5, 60–64. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S, Trent DF, Honey RN, Weir GC (1981) Responses of neonatal rat islets to streptozotocin: limited B‐cell regeneration and hyperglycemia. Diabetes 30 (1), 64–69. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S, Baxter LA, Schupin GT, Smith FA (1993) A second pathway for regeneration of adult exocrine and endocrine pancreas. Diabetes 42 (12), 1715–1720. [DOI] [PubMed] [Google Scholar]
- Bonner‐Weir S, Taneja M, Weir GC, Tatarkiewicz K, Song KH, Sharma A, O’Neil JJ (2000) In vitro cultivation of human islets from expanded ductal tissue. Proc. Natl. Acad. Sci. USA 97 (14), 7999–8004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cras‐Meneur C, Elghazi L, Czernichow P, Scharfmann R (2001) Epidermal growth factor increases undifferentiated pancreatic embryonic cells in vitro. Diabetes 50 (7), 1571–1579. [DOI] [PubMed] [Google Scholar]
- Dichmann DS, Miller CP, Jensen J, Scott Heller R, Serup P (2003) Expression and misexpression of members of the FGF and TGF‐beta families of growth factors in the developing mouse pancreas. Dev Dyn 226 (4), 663–674. [DOI] [PubMed] [Google Scholar]
- Dudek RW, Lawrence IE, Hill RS, Johnson RC (1991) Induction of islet cytodifferentiation by fetal mesenchyme in adult pancreatic ductal epithelium. Diabetes 40 (8), 1041–1048. [DOI] [PubMed] [Google Scholar]
- Duvillie B, Attali M, Aiello V, Quemeneur E, Scharfmann R (2003) Label‐retaining cells in the rat pancreas: location and differentiation potential in vitro. Diabetes 52 (8), 2035–2042. [DOI] [PubMed] [Google Scholar]
- Fell PE, Grobstein C (1968) The influence of extra‐epithelial factors on the growth of embryonic mouse pancreatic epithelium. Exp. Cell Res. 53, 301–304. [DOI] [PubMed] [Google Scholar]
- Ferrand N, Astesano A, Phan HH, Lelong C, Rosselin G (1995) Dynamics of pancreatic cell growth and differentiation during diabetes reversion and STZ‐treated newborn rats. Am. J. Physiol. 269 (5 Part 1), 1250–1264. [DOI] [PubMed] [Google Scholar]
- Finegood DT, Scaglia L, Bonner‐Weir S (1995) Dynamics of β‐cell mass in the growing rat pancreas: estimation with a simple mathematical method. Diabetes 44 (3), 249–256. [DOI] [PubMed] [Google Scholar]
- Garofano A, Czernichow P, Breant B (2000) Impaired β‐cell regeneration in perinatally malnourished rats: a study with STZ. FASEB J. 14 (15), 2611–2617. [DOI] [PubMed] [Google Scholar]
- Gittes GK, Galante PE, Hanahan D, Rutter WJ, Debas HT (1996) Lineage‐specific morphogenesis in the developing pancreas: role of mesenchymal factors. Development 122 (2), 439–447. [DOI] [PubMed] [Google Scholar]
- Golosow N, Grobstein C (1962) Epitheliomesenchymal interactions in pancreatic morphogenesis. Dev. Biol. 4, 242–255. [DOI] [PubMed] [Google Scholar]
- Gomori G (1950) Aldechyde fuchsin: a new stain for elastic tissue. Am. J. Clin. Pathol. 20, 665–666. [PubMed] [Google Scholar]
- Gu D, Sarvetnick N (1993) Epithelial cell proliferation and islet neogenesis in IFN‐γ‐transgenic mice. Development 118, 33–46. [DOI] [PubMed] [Google Scholar]
- Guo L, Degenstein L, Fuchs E (1996) Keratinocyte growth factor is required for hair development but not for wound healing. Genes Dev. 10 (20), 165–175. [DOI] [PubMed] [Google Scholar]
- Guz. Y, Nasir I, Teitelman G (2001) Regeneration of pancreatic β cells from intra‐islet precursor cells in an experimental model of diabetes. Endocrinology 142 (11), 4956–4968. [DOI] [PubMed] [Google Scholar]
- Hardikar AA, Marcus‐Samuels B, Geras‐Raaka E, Raaka BM, Gershengorn MC (2003) Human pancreatic precursor cells secrete FGF 2 to stimulate clustering into hormone‐expressing islet‐like cell aggregates. Proc. Natl. Acad. Sci. USA 100 (12), 7117–7122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart AW, Baeza N, Apelqvist A, Edlund H (2000) Attenuaton of FGF signalling in mouse β– cells leads to diabetes. Nature 408, 864–868. [DOI] [PubMed] [Google Scholar]
- Krakowski ML, Kritzik MR, Jones EM, Krahl T, Lee J, Arnush M, Gu D, Sarvetnick N (1999) Pancreatic expression of keratinocyte growth factor leads to differentiation of islet hepatocytes and proliferation of duct cells. Am. J. Pathol. 154 (3), 683–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larra F, Droz. B (1970) Techniques radioautographiques et leur application a l’etude du renouvellement des constituants cellulaires. J. Microscopie 9, 845–880. [Google Scholar]
- Le Bras S, Miralles F, Basmaciogullari A, Czernichow P, Scharfmann R (1998) Fibroblast growth factor 2 promotes pancreatic epithelial cell proliferation via functional fibroblast growth factor receptors during embryonic life. Diabetes 47 (8), 1236–1242. [PubMed] [Google Scholar]
- Miralles F, Serup P, Cluzeaud F, Vandewalle A, Czernichow P, Scharfmann R (1999) Characterization of β cells developed in vitro from rat embryonic pancreatic epithelium. Dev. Dyn. 214 (2), 116–126. [DOI] [PubMed] [Google Scholar]
- Movassat J, Beattie GM, Lopez. AD, Portha B, Hayek A (2003) Keratinocyte growth factor and beta‐cell differentiation in human fetal pancreatic endocrine precursor cells. Diabetologia 46 (6), 822–829. [DOI] [PubMed] [Google Scholar]
- Ogneva V, Martinova Y (2002) The effect of in vitro fibroblast growth factors on cell proliferation in pancreas from normal and streptozotocin‐treated rats. Diabetes Res. Clin. Pract. 57, 11–16. [DOI] [PubMed] [Google Scholar]
- Plachot C, Portha B (2001) Impaired pancreatic duct‐cell growth in focal areas of regeneration after partial pancreatectomy in the adult Goto‐Kakizaki rat, a spontaneous model of type II diabetes. Histochem. J. 33 (3), 141–147. [DOI] [PubMed] [Google Scholar]
- Portha B, Levacher C, Picon L, Rosselin G (1974) Diabetogenic effect of streptozotocin in the rat during the perinatal period. Diabetes 23 (11), 889–895. [DOI] [PubMed] [Google Scholar]
- Rao MS, Yeldani AV, Reddy JK (1990) Stem cell potential of ductular and periductular cells in the adult rat pancreas. Cell Diff. Dev. 29 (3), 155–163. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Brown R, Duguid W (1983) A new approach to the induction of duct cell epithelial hyperplasia and nesidoblastosis by cellophane wrapping of the hamster pancreas. J. Surg. Res. 35 (1), 63–72. [DOI] [PubMed] [Google Scholar]
- Rosenberg L, Vinik AI, Pittenger R, Rafaeloff R, Duguid WP (1996) Islet‐cell regeneration in the diabetic hamster pancreas with restoration of normoglycaemia can be induced by local growth factor(s). Diabetologia 39 (3), 256–262. [DOI] [PubMed] [Google Scholar]
- Sarvetnick N, Shizuru J, Ligitt D, Martin L, McIntyre B, Gregory A, Parslow T, Stewart T (1990) Loss of pancreatic islet tolerance induced by beta‐cell expansion of interferon‐gamma. Nature 346, 844–847. [DOI] [PubMed] [Google Scholar]
- Scharma A, Zangen DH, Reitz. P, Taneja M, Lissauer ME, Miller CP, Weir GC, Habener JF, Bonner‐Weir S (1999) The homeodomain protein IDX‐1 increases after an early burst of proliferation during pancreatic regeneration. Diabetes 48 (3), 507–513. [DOI] [PubMed] [Google Scholar]
- Vinik A, Pittenger G, Rafaeloff R, Rosenberg L, Duguid W (1996) Determinants of pancreatic islet cell mass: a balance between neogenesis and senescence/apoptosis. Diabetes Rev. 4, 235–263. [Google Scholar]
- Wang R, Kloppel G, Bowens L (2005) Duct‐to islet‐cell differentiation and islet growth in the pancreas of duct‐ligated adult rats. Diabetologia 38, 1405–1411. [DOI] [PubMed] [Google Scholar]
- Wessels NK, Cohen JH (1967) Early pancreatic organogenesis: morphogenesis, tissue interactions, and mass effects. Dev. Biol. 144, 248–261. [DOI] [PubMed] [Google Scholar]
- Xu L, Greisler HP (2002) Angiogenic effect of fibroblast growth factor‐1and vascular endothelial growth factor and their synergism in a novel in vitro quantitative fibrin‐based 3‐dimensional angiogenesis system. Surgery 132 (2), 259–267. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Yasuda M, Hori A, Arishima K, Eguchi Y (2004) Recovery in the fetal pancreatic islet following fetal administration of streptozotocin in the rat in vivo and in vitro . Anat. Rec. A Discov. Mol. Cell. Evol. Biol. 281 (2), 1319–1325. [DOI] [PubMed] [Google Scholar]
- Ye F, Duvillie B, Scharfmann R (2005) Fibroblast growth factors 7 and 10 are expressed in the human embryonic pancreatic mesenchyme and promote the proliferation of embryonic pancreatic epithelial cells. Diabetologia 48, 277–281. [DOI] [PubMed] [Google Scholar]


