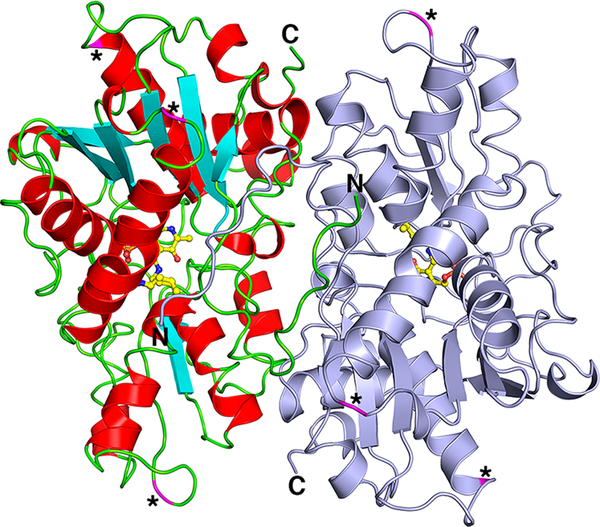
Figure 3.
The yCBS catalytic core (residues 1–353) is a head-to-tail homodimer. One monomer is colored light blue, and the other monomer is colored according to secondary structure, in which α-helices are red, β-strands are cyan, and loop regions are green. PLP and the covalently linked K53 are shown in ball-and-stick with carbon atoms colored yellow. Asterisks and magenta-colored ribbons indicate locations of sequence insertions of yCBS residues (residues 74, 181–185, and 304–306; Figure S1) relative to the hCBS and dCBS protein sequences. There is one cis-proline in the structure, at Q229-P230, which forms a tight turn between a strand and the following short α-helix. All of the structures reported here show this same cis-proline configuration. A comparison of the overall structures of all of the yCBS-cc structures reported here is shown in Figure S5. No major rearrangements are observed.