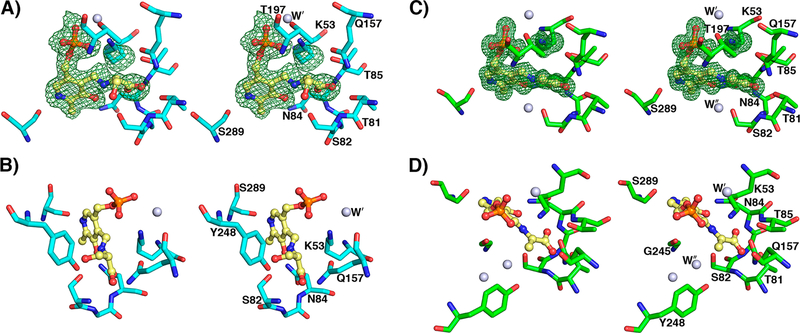
Figure 5.
yCBS-cc active site intermediates. (A) The PLP–l-serine intermediate active site at pH 8.0. K53 is shifted away from PLP, and the pyridine ring is tilted away from its position in the apoenzyme by about 11° (Figure S8). The Fobs – Fcalc electron density contoured in green at 3σ from a simulated annealing omit map supports the external aldimine intermediate modeled into the active site. (B) The hydroxyl group of S82 is within hydrogen-bonding distance of the β-OH leaving group of the l-serine intermediate. The carboxylate group of the intermediate makes similar interactions as the acetate noted in the internal aldimine yCBS-cc active site (Figure 4). (C) The PLP–l-aminoacrylate intermediate active site at pH 6.5. K53 is shifted away from PLP. The carboxylate group of the intermediate makes similar interactions as the acetate noted in the internal aldimine yCBS-cc active site (Figure 4). (D) A water in the active site indicates the expected position of the attacking sulfur on the substrate. This water is also noted in the internal aldimine active site (Figure 4) but is absent in the E-PLP-l-ser intermediate active site in (A), where it is displaced by the β-OH group of the serine moiety, and in the PMP complex active site (Figure 7), where lower-resolution electron density does not support placing it there. Atoms are colored according to element as follows: cyan or green, carbon; blue, nitrogen; red, oxygen; orange, phosphorus. The carbon atoms of PLP are colored yellow.