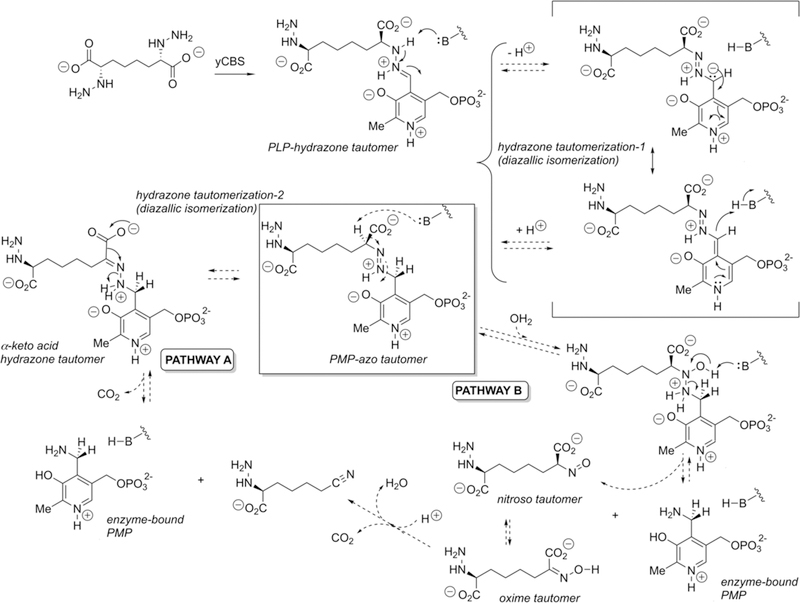
Figure 6.
Potential mechanisms for tautomerization and subsequent fragmentation of the PLP hydrazone expected to form from the designed hydrazine inhibitor and the internal aldimine form of the CBS enzyme. The crystallographically observed reduced form of the cofactor (PMP), under redox-neutral conditions, suggests that the initial PLP hydrazone may tautomerize to the azo form, which itself could undergo N–N cleavage following a second tautomerization and decarboxylation (pathway A) or via hydration and disproportionation (pathway B), at least at sufficient levels to result in significant active-site occupancy by the PMP form of the cofactor. Although the pyridine nitrogen appears as protonated in this figure, there is some question about its state of protonation (Figure 2).