Fig. 1.
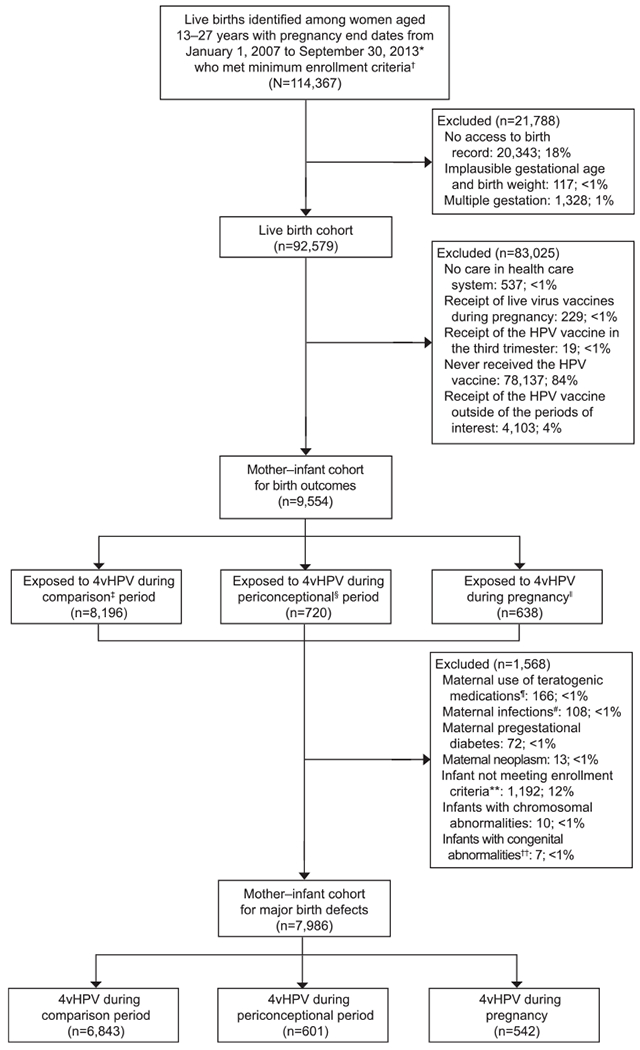
Flowchart. 4vHPV, quadrivalent human papillomavirus (HPV) vaccine. *Except for one site, January 20, 2009–September 30, 2013. †Continuous insurance enrollment from 6 months before their last menstrual period through 6 weeks postpartum. ‡Eighteen to 4 months before pregnancy. §Two weeks before to 2 weeks after last menstrual period. ‖Two to 28 weeks of gestation. ¶Isotretinoin, bexarotene, acitretin, misoprostol, methotrexate, mycophenolate mofetil, azathioprine, thalidomide, warfarin, lithium, amiodarone, dronedarone, carbamazepine, fosphenytoin, phenytoin, mephobarbital, phenobarbital, primidone, topiramate, valproate, leflunamide. #Toxoplasmosis, syphilis, varicella, rubella, and cytomegalovirus in mother’s chart. **Infants surviving to age 1 year; enrollment consists of 4 non-consecutive months during the first year of life, at least 1 month of insurance in the first 3 months of life, and at least one care encounter. Infants who died during the first year of life and those hospitalized for more than 30 days after birth were not required to meet criteria. Twelve percent of infants did not meet enrollment criteria: 10.7% of during-pregnancy exposures, 12.1% of exposures during the periconceptional period, and 12.9% of the comparison group for these birth defect analyses. ††Toxoplasmosis, syphilis, varicella, rubella, and cytomegalovirus in infant chart.
