Figure 2.
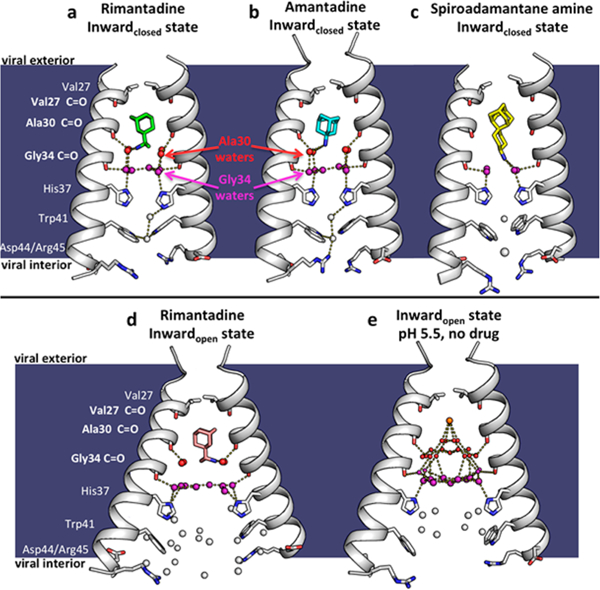
X-ray crystal structures of the M2 proton channel bound to drugs and inhibitors. The channel is a homotetramer, but here the front and back monomers have been removed to show the contents of the channel pore. Hydrogen bonds are shown as yellow dashes. The layer of waters forming hydrogen bonds to the Ala30 carbonyls (“Ala30 layer”) is shown as red spheres; the layer of waters forming hydrogen bonds to the Gly34 carbonyls (“Gly34 layer”) is shown as purple spheres. Top, left to right: (a) M2 bound to rimantadine in the Inwardclosed state (PDB code 6BKL, 2.00 A resolution, monomer subunits F and H); (b) M2 bound to amantadine in the Inwardclosed state (6BKK, 2.00 Å resolution, monomers B and D); (c) M2 bound to spiro-adamantyl amine in the Inwardclosed state (6BMZ, 2.63 Å resolution, monomers B and D). Bottom, left to right: (d) M2 bound to rimantadine in the Inwardopen state (6BOC, 2.25 Å resolution, monomers B and D); (e) Previously solved structure of M2 in the Inwardopen state at pH 5.0 in the absence of bound drug31 (5JOO, 1.41 Å resolution).
