Scheme 1.
Short enantioselective synthesis of azamerone.a
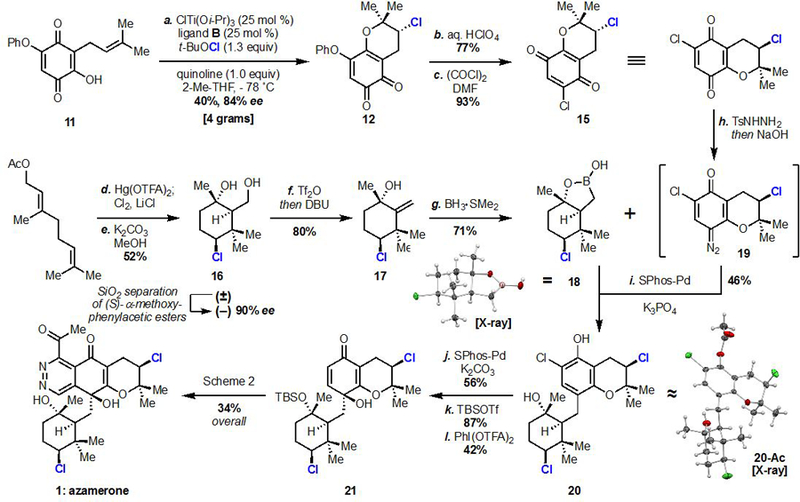
a Reagents and conditions: (a) ClTi(Oi-Pr)3 (0.25 equiv), B (0.25 equiv), quinoline (1.0 equiv), t-BuOCl (1.3 equiv), 2-Me-THF, –78 ˚C, 40%, 84% ee; (b) aq. HClO4 (1.3 equiv), Et2O, 77%; (c) oxalyl chloride (1.1 equiv), DMF (1.4 equiv), MeCN, 0 ˚C, 93%; (d) Hg(OTFA)2 (1.1 equiv), MeNO2; Cl2, LiCl (3.0 equiv), pyridine; (e) K2CO3, MeOH, 50 ˚C, 52% overall; (f) Tf2O (1.05 equiv), 2,6-lutidine (1.2 equiv), then DBU (2.5 equiv), DCM, –78 ˚C to RT, 80%; (g) BH3·SMe2 (2.0 equiv), THF, 0 ˚C to RT, 71%; (h) TsNHNH2 (1.1 equiv), MeOH, then 1M aq. NaOH, DCM; (i) (SPhos)Pd-G3: (SPhos)[2-(2′-amino-1,1′-biphenyl)]palladium(II) methanesulfonate (0.1 equiv), K3PO4 (1.3 equiv), dioxane, 60 ˚C, 46% over 2 steps, 10:1 dr; (j) (SPhos)Pd-G3 (0.1 equiv), K2CO3 (2.0 equiv), i-PrOH, 90 ˚C 56%; (k) TBSOTf (3.0 equiv), (i-Pr)2NEt (4.0 equiv), DCM then 3M aq. NaOH (7.5 equiv), i-PrOH, 87%; (l) PhI(OTFA)2 (1.1 equiv), 3:1 MeCN/H2O, 42%.
