Table 1.
Enantioselective chloroetherification optimization
 | |||
|---|---|---|---|
| entrya | conditions | yield (%) | ee (%) |
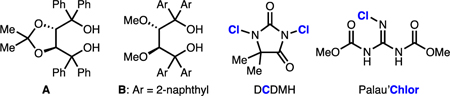
| 1 | A, t-BuOCI, DCM | 29 | 10 |
| 2 | B, t-BuOCI, DCM | 39 | 7 |
| 3 | B, t-BuOCI, hexanes | 11 | <1 |
| 4 | B, t-BuOCI, PhMe | 38 | 16 |
| 5 | B, t-BuOCI, Et20 | 36 | 14 |
| 6 | B, t-BuOCI, t-BuOMe | 25 | 16 |
| 7 | B, t-BuOCI, THF | 18 | 22 |
| 8 | B, t-BuOCI, 2-Me-THF | 33 | 57 |
| 9 | B, NCS, 2-Me-THF | <5 | - |
| 10 | B, DCDMH, 2-Me-THF | 42 | 33 |
| 11 | B, Palau’Chlor, 2-Me-THF | 28 | 29 |
| 12 | B, t-BuOCI, 2-Me-THF, pyridine | 20 | 78 |
| 13 | B, t-BuOCI, 2-Me-THF, quinoline | 24 | 81 |
| 14b | B, t-BuOCI, 2-Me-THF, quinoline | 68 | 84 |
| 15c | B, t-BuOCI, 2-Me-THF, quinoline | 45 | 79 |
| 16c,d | B, t-BuOCI, 2-Me-THF, quinoline | 40e | 84 |
 | |||
Reactions were conducted on 0.035–0.176 mmol scale, and 1H-NMR yields are reported based on 1,4-dinitrobenzene as internal standard;
100 mol % ClTi(Oi-Pr)3, 100 mol % B
1.3 equiv t-BuOCl
reaction conducted on 4.0 grams 11
isolated yield.
