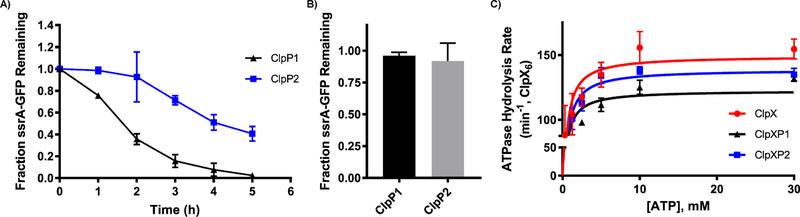
Figure 6.
ClpXP mediated degradation of ssrA-GFP and ClpP induced reduction in ATP hydrolysis. (A) ClpXP1 and ClpXP2 mediated degradation of ssrA-GFP. Experiment was performed in triplicate, and SDS-PAGE results were quantified with ImageJ (NIH) to provide the quantified results (graph). (B) Fraction of ssrA-GFP remaining when incubated with ClpP variants in the absence of ClpX. (C) Rate of C. difficile ClpX ATP hydrolysis in the presence or absence of C. difficile ClpP1 or ClpP2. C. difficile ClpX hydrolyzed ATP at a rate of 149.2 ± 6.0 min−1·ClpX6−1. In the presence of ClpP1, ClpX hydrolyzed ATP at a rate of 122.5 ± 0.3 min−1·ClpX6−1. In the presence of ClpP2, ClpX hydrolyzed ATP at a rate of 138.7 ± 0.4 min−1·ClpX6−1. Km values for ClpX, ClpXP1, ClpXP2 are 375.2, 318.9, and 402.9 μM, respectively.