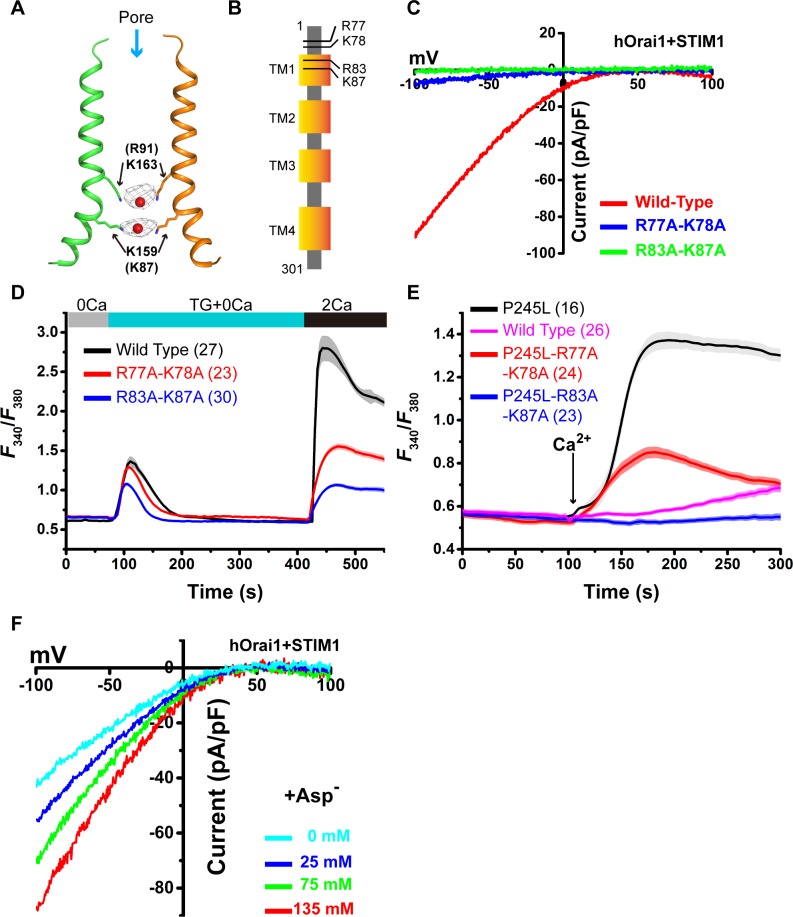
Fig 4. Anions facilitate Ca2+ permeation.
(A) Anion binding in the pore. The 2Fo-Fc Fourier electron density map contoured at 2.0 σ for anions is shown as a gray mesh. TM1 helices from 2 opposite subunits are depicted. Residues K163 and K159 are shown as sticks, and anions are shown as red spheres. (B) Schematic model of the hOrai1 channel. The positions of 4 basic residues (R77, K78, R83, and K87) are indicated. (C) Representative I–V curves of the whole-cell Ca2+ currents of STIM1-activated wild-type hOrai1 and mutants. (D) Extracellular Ca2+ influx in HEK-293T cells co-expressing STIM1-YFP and wild-type or mutant hOrai1-GFP. (E) Extracellular Ca2+ influx in HEK-293T cells expressing wild-type or mutant hOrai1-GFP. (F) Representative I–V curves of the whole-cell Ca2+ currents of STIM1-activated wild-type hOrai1 at the indicated concentration of cesium aspartate. Primary data can be found in S1 Data. Asp, aspartate; GFP, green fluorescent protein; HEK, human embryonic kidney; hOrai1, human Orai1; STIM, stromal interaction molecule; TM, transmembrane; YFP, yellow fluorescent protein.