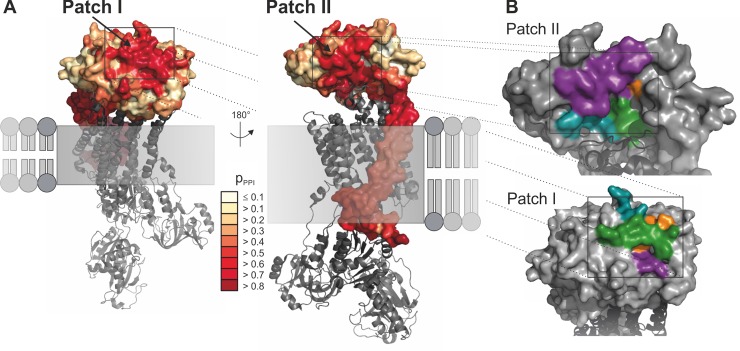
Fig 3. Localization and composition of putative protein-protein interaction sites.
(A) The putative protein-protein interaction sites identified by PresCont for a homology model of ATP1B2 are colored according to the pPPI score, which is for each residue the probability that it contributes to a protein-protein interaction site. The membrane-bound domain of ATP1B2 is composed of hydrophobic residues, which causes false positive predictions. The localization of the α subunit of the Na/K-ATPase complex from S. acanthias (PDB-ID 3a3y, in grey) results from a superposition of the corresponding β subunits. (B) Detailed view of patch I (lower panel) and II (upper panel). The four regions forming the patch are colored in cyan (region 1), purple (region 2), green (region 3), and orange (region 4). The position of the α subunit colored in dark grey was transferred from the Na/K-ATPase complex of S. acanthias (PDB-ID 3a3y) by means of superposition.