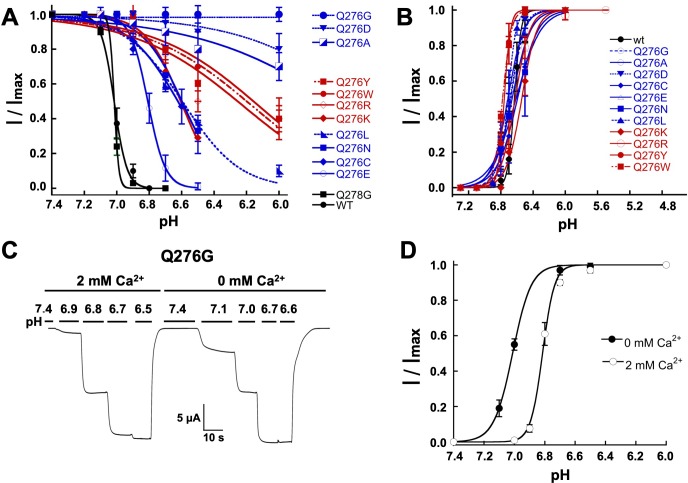
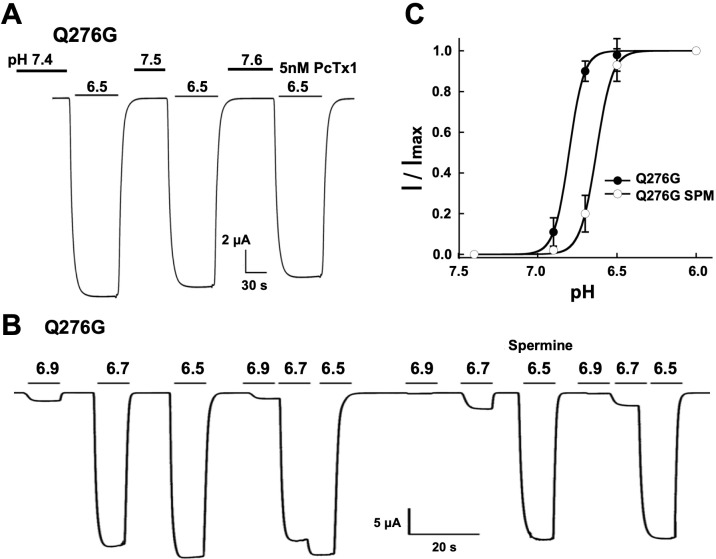
Figure 3. Mutations of Q276 diminish SSD.
(A) Values of apparent pHSSD and (B) apparent pH50A of substitutions in position 276. Curves represent fits of data points (n = 5–8 independent measurements) to the Hill equation. (C) Current traces of Q276G activated sequentially with solutions of the indicated pH (bars above the trace) in the presence of 2 mM Ca2+ and nominal 0 mM Ca2+. Pre-conditioning protons do not induce SSD in any of the two conditions. (D) The absence of Ca2+ in the activating solution increases the apparent affinity for protons from pH50A6.8 to 7.0.