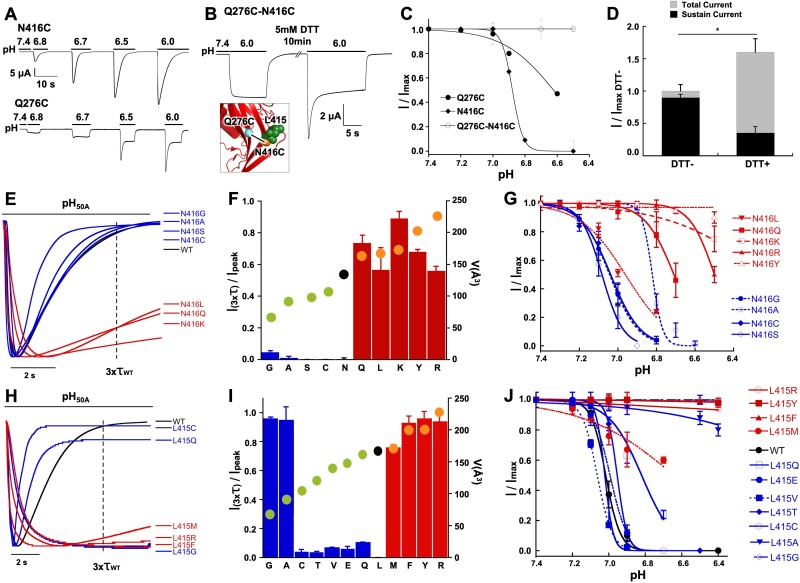
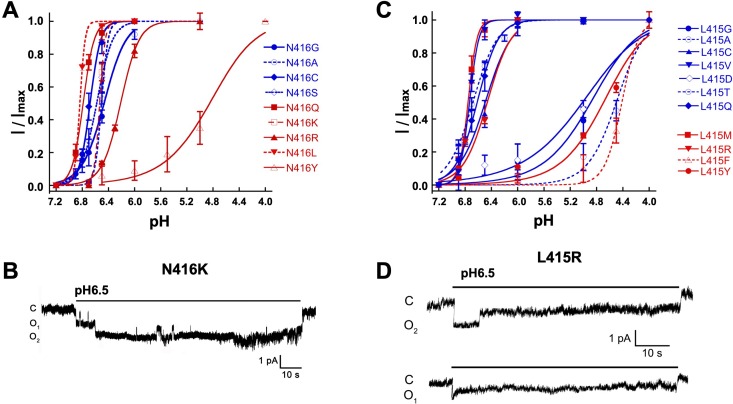
Figure 5. Functional and physical interactions of Q276 and N416 residues.
(A) Current traces of single cysteine mutants N416C and Q276C activated by increasing concentrations of protons. N416C channels completely desensitize, whereas Q276C exhibit a fraction of sustained current. (B) The double mutant Q276C-N416C exhibits only sustained currents. After treatment with DTT a desensitizing current appeared. Inset shows predicted locations of the two cysteines in the closed conformation and a putative disulfide bond as a line linking the residues. (C) Comparison of the pH dependence of SSD of single and double mutants shows the double mutant is insensitive to SSD. (D) Columns represent the fractions of sustained and peak currents before and after DTT treatment. Currents were normalized to the values prior to DTT; n = 15 cells. Asterisks indicate p values < 0.005. (E) Normalized currents of substitutions of N416 superimposed on the wild type (black trace). (F) Ratio of sustained/peak currents at time 3xτwt of N416 mutants ordered from low to large side chain volume indicated by the filled circles on the bars. Error bars are SD, n = 8 to 10 cells. (G) pH dependence of SSD of N416 mutants. Lines are the fit of data points (n = 5–8 independent measurements) to the Hill equation ± SD. (H) Normalized currents of substitutions of L415 superimposed on the wild type (black trace). (I) Ratio of sustained/peak currents at time 3xτwt of L415 mutants ordered from low to large side chain indicated by the filled circles on the bars. Error bars are SD, n = 8 to 10 cells. (J) pH dependence of SSD of L415 mutants. E data point is the mean ± SD of 5 to 8 independent cells.