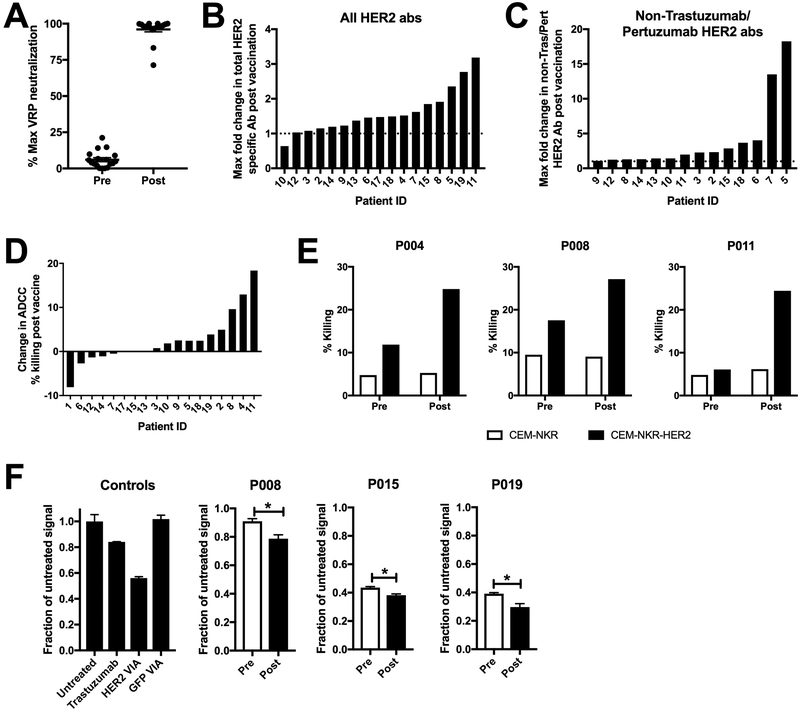
Figure 3. Induction of poly-functional VRP and HER2-specific antibodies following vaccination.
A.) Patient serum pre and post VRP-HER2 vaccination was tested for VRP vector specific antibodies in a VRP neutralization assay. VRP vector expressing a control transgene was incubated with patient serum and plated with Vero cells. Expression of transgene was determined by staining of cells with fluorochrome labeled antibody for transgene and analyzed by flow cytometry. B and C.) Serum was analyzed for HER2-specific antibodies using a cell-based ELISA. Cells expressing full length HER2 (B) or cells expressing a mutated form of HER2 that is not bound by Trastuzumab or Pertuzumab (C) were used. Absorbances are corrected for signal seen in a negative control non HER2 expressing plate. Several dilutions were assessed and the maximum observed fold change in the level of HER2-specific antibody signal post-vaccination for each patient is reported. If values were below the level of detection for a particular patient, they are not shown. D.) CEM.NKR cell line expressing HER2 (targets) were incubated with serum from patients vaccinated with VRP-HER2 and normal donor PBMC (effectors) at an E:T ratio of 1:25 in an ADCC assay for 2-4 hours. Cells were stained with annexinV and 7-AAD to determine total lysis of CEM.NKR-HER2 targets. Shown as percent change in maximum killing resulting from serum pre verses post VRP-HER2 vaccination. E.) Individual killing results for patients 4, 8, and 11 from (D) are shown. F.) internalization of HER2 was measured using a Mars1-tagged form of HER2. Background signal from non-HER2 expressing cells was subtracted. Level of Mars1 signal was then normalized to untreated cells. VIA= Vaccine-induced antibodies. Individual results from patients 8, 15, and 19 are shown. *p<0.05