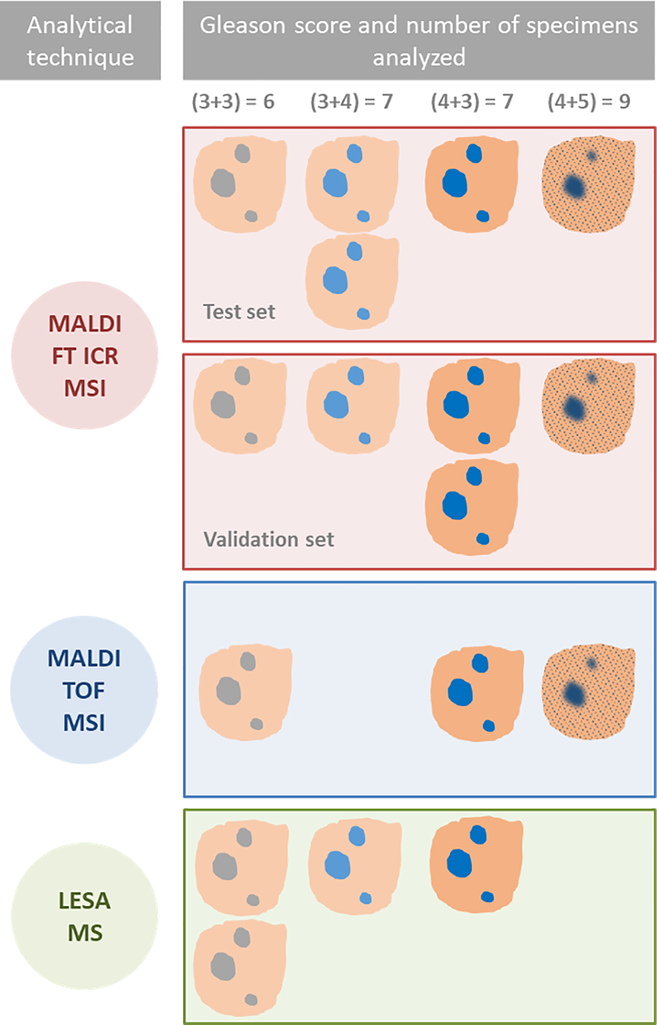
Fig. 2.
Schematic description of experimental design and specimens used for each study; MALDI FT ICR MSI tissue imaging was used to characterize lipids and metabolites in tumor and normal tissues and assess differences between different Gleason scores (GS) in a test set of five specimens. MALDI FT ICR MSI was performed on a second set of five specimens consisting of the same range of GS for validation. The method was translated using two faster MS based techniques, MALDI TOF MSI and LESA MS. These were performed on a further three and four specimens respectively, to validate the findings by MALDI FT ICR MSI and demonstrate the feasibility of a more rapid, MS method to characterize normal and tumor tissue in situ.