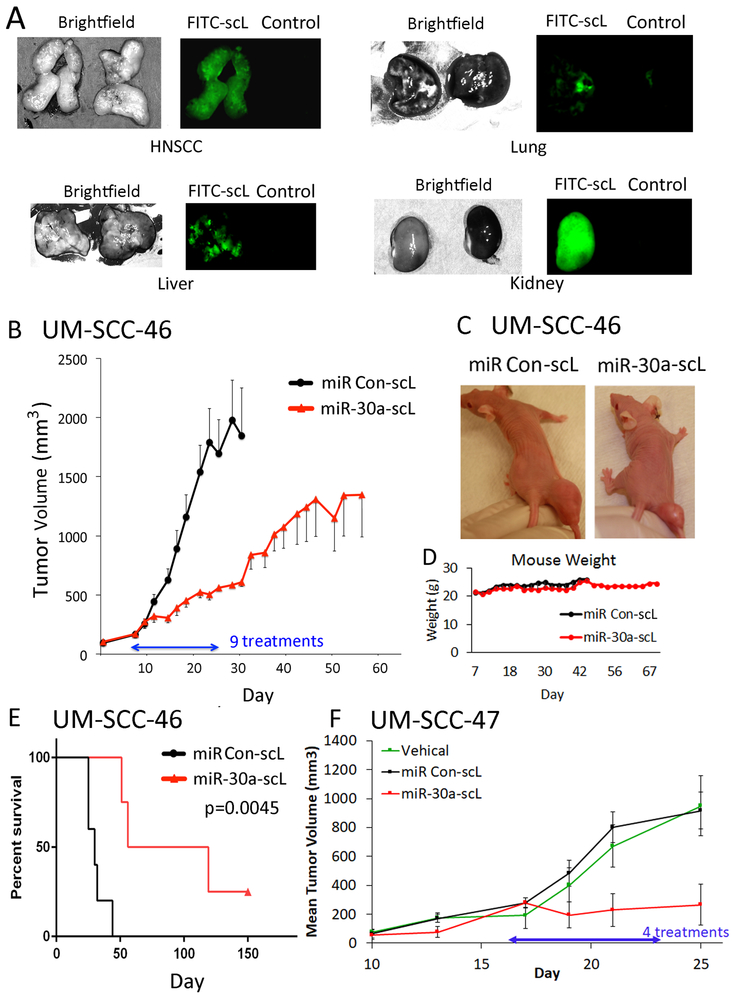
Fig. 5. Anti-tumor activity of miR-30a-5p mimic delivered via nanoparticles in HNSCC xenograft tumors in vivo.
(A) Detection of FITC-labeled-scL in tumor and selected organ tissues. UM-SCC-46 cells were subcutaneously injected into athymic nu/nu female mice, and mice were randomized when tumors reached ~300 mm3. Mice were administered 100 μg (~5 mg/kg) of complexed FITC-labeled control oligonucleotide or control vehicle intravenously (IV). 24 h later, mice were sacrificed for tumor and organ harvest. Dissected organs were then imaged. Left panels, bright field; right panels, fluorescence microscopy, where tissues with FITC nanoparticles are on the left with green fluorescence and control vehicle on the right. (B) Effect of miR-30a-5p mimic on tumor xenogafts. Mice bearing ~150 mm3 UM-SCC-46 xenograft tumors were randomized into groups of 5 animals and injected IV with nine doses of 60 µg (~3 mg/kg) of miR-30a-5p mimic nanoparticles (miR-30a-scL) or control nanoparticles on Monday, Wednesday, and Friday (MWF) for 3 weeks. Mean tumor volume for each group ±SEM; statistically significant size difference between the two groups was achieved by day 16 (p<0.05, student’s t-test). (C) Representative images of tumor size at the end of treatment on day 24. (D) Mouse weight (grams) over course of the experiment. (E) Kaplan-Meier analysis plot showing significant improvement in survival between mice treated with miR-30a-scL vs. control. (F) Mice bearing HPV+ UM-SCC-47 xenograft tumors grown to ~150 mm3, were randomized into groups of 4, and then the mice were injected IV with 60 µg miR-30a-scL or control on an MWF schedule for 25 days. Tissue was collected 36 hrs after the final dose. Mean tumor volume ± SEM, * p < 0.05, student’s T-test on day 25 after tumor implant.