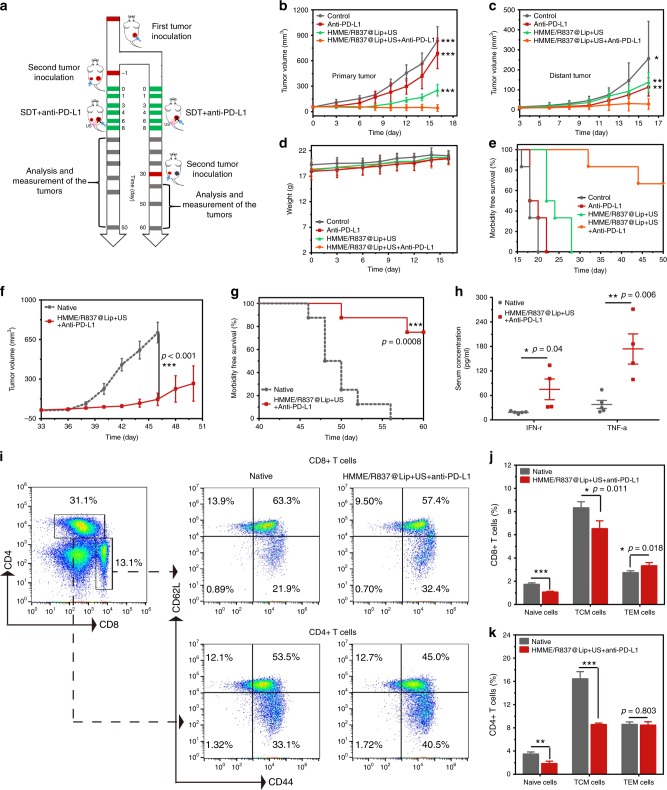
Fig. 8.
Anticancer activity of HMME/R837@Lip-augmented SDT plus anti-PD-L1 therapy in murine colorectal cancer models. a Schematic illustration of the experiment design to assess the antitumour immune responses against mimic distant tumours and the immunological memory response triggered by HMME/R837@Lip-augmented SDT and anti-PD-L1 combination therapy; b, c primary (b) and mimic distant (c) tumour growth curves of different groups of tumour-bearing mice after various treatments as indicated in the figure. Error bars are based on SD (n = 6); d time-dependent body-weight surveillance of mice (n = 6) after different treatments; e morbidity-free survival of mice after different treatments (n = 6); f tumour-growth curves of the rechallenged tumours in the corresponding groups were stopped when the first mouse died. Error bars are based on SE (n = 8); g morbidity-free survival of mice after the indicated treatment (n = 8), statistical significance was calculated via the log-rank (Mantel–Cox) test; h TNF-α and IFN-γ levels in serum isolated from mice of the treatment group and native group 7 days after the second tumour was introduced. Error bars are based on SE (n = 5 for the native group, n = 4 for the combined treatment group); i representative flow-cytometry plots of splenic lymphocytes of CT26 tumour-bearing mice treated with the combined immunotherapy at day 29 right before mice rechallenged with the secondary tumours (gating on CD3+ cells); j, k absolute quantification of naive T cells, TCM and TEM in the spleen. TCM central memory T cells, TEM effector memory T cells. Error bars are based on SD (n = 4). Statistical significances were calculated via Student’s t test. *p < 0.05, **p < 0.01 and ***p < 0.001