Abstract
Context
In children with isolated growth hormone (GH) deficiency (GHD), routine biochemical screening for multiple pituitary hormone deficiencies (MPHD) and adverse effects related to growth hormone (GH) treatment are frequently performed. More evidence is needed to support this practice.
Objectives
To evaluate the rate of development of MPHD among children initially diagnosed with isolated GHD and to assess the utility of screening tests to identify complications of GH therapy.
Design
Retrospective analysis of subjects treated with GH since 2005. For the first objective, only subjects diagnosed with GHD were included. Subjects were excluded if GHD was associated with an acquired disorder or condition known to be associated with pituitary abnormalities. For the second objective, other GH-treated diagnoses were included.
Patients
A total of 328 subjects (171 with GHD, 154 with idiopathic short stature, and three with SHOX deficiency).
Results
In subjects with isolated GHD, MPHD was diagnosed in seven (4.2%) after a mean of 35.4 months (range, 9.4 to 68.0). Sex, age at diagnosis, duration of GH, and peak stimulated GH levels were not associated with developing MPHD. Among subjects with an MRI abnormality, 13.9% developed MPHD (OR, 6.3; 95% CI, 1.2 to 33.7). In the entire cohort, three subjects (0.9%) developed dysglycemia, and no subject had persistently abnormal liver or renal function tests.
Conclusions
There is a limited role for routine biochemical screening for MPHD in children with idiopathic isolated GHD or for adverse effects in otherwise healthy children. Routine biochemical screening for MPHD should be limited to those with an abnormal MRI.
Keywords: growth hormone deficiency, multiple pituitary hormone deficiency, screening tests, children
Growth hormone (GH) deficiency (GHD) in children results from the inability of the pituitary gland to produce sufficient GH to maintain glucose homeostasis and/or to achieve normal growth velocity. GHD has traditionally been diagnosed by the presence of a suboptimal GH response during stimulation testing, prompted by an evaluation for short stature or decreased growth velocity. GHD can be isolated or can occur in the context of multiple pituitary hormone deficiencies (MPHD). There are many organic causes of GHD, including brain tumors, trauma, surgery, radiation, and genetic abnormalities. However, GHD is often not associated with such conditions and is instead termed “idiopathic.”
As part of the clinical management of children with GHD, routine screening laboratory tests are often requested. These tests are done in children with isolated GHD to monitor for the development of additional pituitary hormone deficiencies and in these and other children on GH to screen for possible adverse effects associated with GH treatment. Children with GHD due to an organic cause are at high risk of developing additional pituitary hormone deficiencies and should be routinely tested for the development of other abnormalities [1]. Children with idiopathic GHD are also often routinely screened for the development of MPHD; however, the available evidence indicates that the rationale for screening in this population is less clear. Likewise, what “safety” laboratories should be done to screen for adverse effects of treatment has not been clearly established. For this, the rationale is probably strongest for dysglycemia screening as outlined in the Pediatric Endocrine Society GH guidelines [2], but other tests are sometimes done as well, including assessments for renal and hepatic injury. Even for dysglycemia, although it is apparent that children on GH with underlying risk factors for the development of insulin resistance should be monitored [2–5], it is less clear whether screening is warranted in all children on GH. Given the common use of GH to treat patients in pediatric endocrinology practices, it is conceivable that a large amount of unnecessary laboratory testing may be done.
The reported rate of the development of MPHD in children with idiopathic isolated GHD ranges from 2% to 44%. An observational trial using data from the Genetics and Neuroendocrinology of Short Stature International Study (GeNeSIS) found that only 2.0% of children with isolated idiopathic GHD developed MPHD [6]. This study relied on data reported at the discretion of local investigators and included subjects with a normal MRI as well as subjects in whom MRI results were not available. Tsai et al. [7] examined the records of 52 subjects with isolated idiopathic GHD and found an overall rate of the subsequent development of MPHD to be 28.8%, but the rate was only 3.8% in children with a normal MRI. However, in this report there was no description of how hormone deficiencies were defined, what deficiencies developed, or the timeframe in which they appeared. The longest follow-up study of children with isolated idiopathic GHD demonstrated a much higher rate of eventual MPHD of 44.6% (37/83 subjects) [8].
Large observational cohort studies have suggested that children treated with GH have an increased risk for impaired glucose tolerance and type 2 diabetes mellitus compared with the general population [3–5]. In each study, dysglycemia was most frequently found in subjects with underlying risk factors (e.g., obesity, malignancy, or Turner syndrome) and less frequently in otherwise healthy children with isolated idiopathic GHD. Therefore, it is not clear if nonobese and otherwise healthy children on GH treatment warrant regular routine screening.
More research is required to determine the need for routine laboratory screening in children treated with GH. In this study, we assessed the utility of commonly performed tests among children with isolated idiopathic GHD by evaluating data from 328 subjects in our clinic.
1. Materials and Methods
We reviewed the charts of all children started on GH at the Hospital for Sick Children in Toronto, Canada, between January 2005 and April 2016. Our study had two aims. The first was to examine the occurrence of other pituitary abnormalities among children and youth initially diagnosed with isolated idiopathic GHD. Subjects were included in the isolated idiopathic GHD cohort if diagnosed with GHD due to a suboptimal response to stimulation testing using clonidine, arginine, and/or glucagon. The stimulated GH cutoff level for diagnosing GHD in our program was <8.0 ng/mL until 29 September 2011; after that date standard preparations using IS 98/574 assay were introduced, and the cutoff level was accordingly changed to <5.7 ng/mL. This change was made because the cutoff of 5.7 ng/mL using the new standards was found to be equivalent to the previous 8.0 ng/mL value based on assessment of multiple duplicate samples analyzed by Passing-Bablok regression. For this paper, all GH levels were adjusted to reflect those found in the most recent assay to ensure comparable values were used in analyses throughout the study. Because we specifically analyzed the rate of development of additional pituitary hormone deficiencies in children with idiopathic isolated GHD, subjects were excluded if (i) GHD was due to an organic cause, such as neoplasm, radiation, or trauma; (ii) GHD was associated with a syndrome known to increase the risk of MPHD or other medical problems, such as Prader-Willi syndrome or septo-optic dysplasia; or (iii) if the subject had MPHD at the time of GH initiation. Subjects were also excluded if they had a comorbid chronic disease, such as inflammatory bowel disease or systemic lupus erythematosus.
The second aim was to assess the utility of screening tests used to identify complications of GH therapy. For this portion of the study, we expanded the population to include children with short stature homeobox (SHOX) deficiency, confirmed on genetic testing, or idiopathic short stature (ISS). ISS was defined as short stature where other known causes had been excluded, a height less than −2.25 SD below the mean for age and sex, an annual height velocity below the 25th percentile for bone age and sex, and an estimated final height below the third percentile. Subjects were excluded if they had a comorbid chronic illness because additional regular blood work screening for the management of this comorbid condition could not be distinguished from screening for adverse effects related to GH treatment and because any abnormalities, if found, might not be attributable to GH administration.
Auxiological data at the time of GH start were collected. Height and body mass index [weight (kg)/height (m2)] z scores were calculated using reference data provided by the World Health Organization standardized reference charts. Growth velocity z scores were generated from published data [9]. Length of follow-up was determined to be the time from when GH was initiated until the subject’s last clinic visit prior to the end of the study period, until they discontinued GH treatment, or until the last clinic visit while on GH therapy if the subject moved away or was lost to follow-up. In the GHD cohort, 87 out of 171 subjects (51%) completed treatment prior to the study end date.
Central hypothyroidism, hypogonadotropic hypogonadism, ACTH deficiency, or central diabetes insipidus was determined to be present or diagnosed once the subject was started on corresponding hormone replacement by the treating pediatric endocrinologist.
X-rays were read by a pediatric radiologist according to the standards of Greulich and Pyle [10]. MRI results were included only if the scan was done at our institution. All MRIs were reviewed by a pediatric radiologist with specialized training in neuroradiology. MRIs were classified as abnormal if a note was made in the report of any anomaly of hypothalamus and/or pituitary gland.
Standard practice within our clinic is to use initial GH doses of 0.03 mg/kg/d for children with GHD and 0.05 mg/kg/d in children with ISS and SHOX deficiency. Doses are then titrated based on clinical response and to maintain the IGF-1 level below the upper limit of normal.
Results were expressed as mean ± SD or median (range). Differences in measurements between diagnostic groups were assessed with independent sample t tests for normally distributed data and Mann-Whitney or χ2 tests for data that were not normally distributed. The association of clinical variables to the development of MPHD was analyzed using ORs with 95% CIs given the low event rate of developing additional pituitary hormone deficiencies. Time to progression of additional pituitary hormone deficiencies was demonstrated by the Kaplan-Meier curve.
2. Results
A. Cohort Characteristics
A total of 328 subjects were included in study cohort. Reasons for GH treatment were idiopathic isolated GHD in 171 (52%), ISS (including small for gestational age) in 154 (47%), and SHOX deficiency in three (1%). The total cohort represents 1154 patient years on GH. Characteristics of the total cohort, as well the GHD and ISS/SHOX deficiency subsets, are provided in Table 1. A total of 114 of 123 subjects with GHD, in whom relevant data were available, were primed with sex steroids as per our protocol to prime patients with Premarin (1.25 mg;Pfizer Canada Inc.) for the two nights prior to testing if their bone age was >8 years and if they were Tanner stage 1 or 2. Eighty-five percent of subjects underwent growth hormone stimulation testing using arginine and clonidine, 8% with arginine and glucagon, and 7% with arginine alone. Mean peak stimulated GH level in the GHD cohort was 3.7 ± 1.4 µg/L.
Table 1.
Cohort Characteristics at Time of GH Start and Duration of GH Treatment
| Total Cohort (n = 328) | GHD (n = 171) | ISS/SHOX (n = 157) | P Valuea | |
|---|---|---|---|---|
| Female, % | 34 | 33 | 34 | 0.85 |
| Age, y | 11.1 (0.3–17.8) | 10.8 (0.3–17.8) | 11.4 (2.4–16.3) | 0.16 |
| Height z score | −2.68 ± 0.96 (−2.67) | −2.67 ± 1.01 (−2.60) | −2.70 ± 0.89 (−2.72) | 0.75 |
| BMI z score | 0.27 ± 1.35 (0.31) | 0.68 ± 1.31 (0.73) | −0.21 ± 1.24 (−0.31) | <0.01 |
| Growth velocity z score | −0.60 ± 1.44 (−0.61) | −0.64 ± 1.85 (−0.50) | −0.57 ± 1.06 (−0.71) | 0.71 |
| IGF-1, µg/L | 200.7 ± 109.9 (180) | 164.2 ± 94.0 (149) | 243.6 ± 113.6 (189) | <0.01 |
| Bone age minus chronological age, y | −1.2 ± 1.4 (−1.1) | −1.6 ± 1.2 (−1.6) | −0.9 ± 1.4 (−0.8) | <0.001 |
| Duration of GH treatment, mo | 37.0 (2.7–131.6) | 37.3 (2.7–131.6) | 36.7 (3.3–121.0) | 0.63 |
Data expressed as percentage, median (range), or mean ± SD (median).
Abbreviations: BMI, body mass index.
GHD compared with the ISS/SHOX cohort.
B. MPHD Among Subjects With GHD
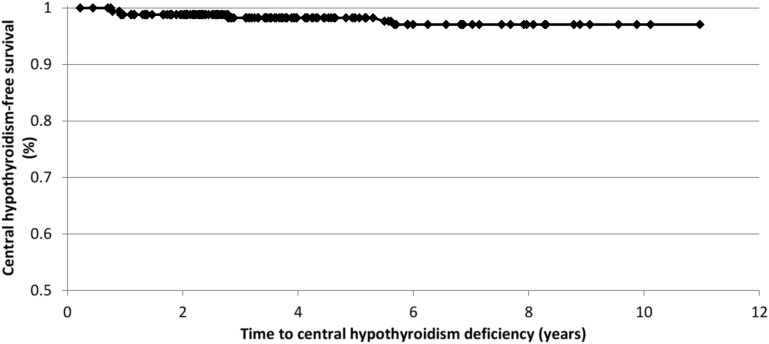
Of the 171 subjects with initial diagnosis of isolated GHD, MPHD developed in seven subjects (4.1%). Five subjects developed central hypothyroidism, three developed hypogonadotropic hypogonadism, and one subject developed both. The diagnosis of central hypothyroidism was made in all five patients after the finding of low free T4 concentrations with inappropriately normal TSH levels. Hypogonadotropic hypogonadism diagnoses were made on the basis of a combination of a lack of pubertal progression after 14 years of age in all three subjects and either a low basal or a stimulated LH concentration. No cases of ACTH deficiency or diabetes insipidus were found. The first case of MPHD occurred 9.4 months after GH start, and no MPHD developed after 68 months. Figure 1 shows the rate of development of central hypothyroidism over time. There was no significant difference in age, sex, duration of GH treatment, or peak stimulated GH level in those who did or did not develop MPHD.
Figure 1.
Rate of development of central hypothyroidism in patients with initial diagnosis of idiopathic, isolated GHD.
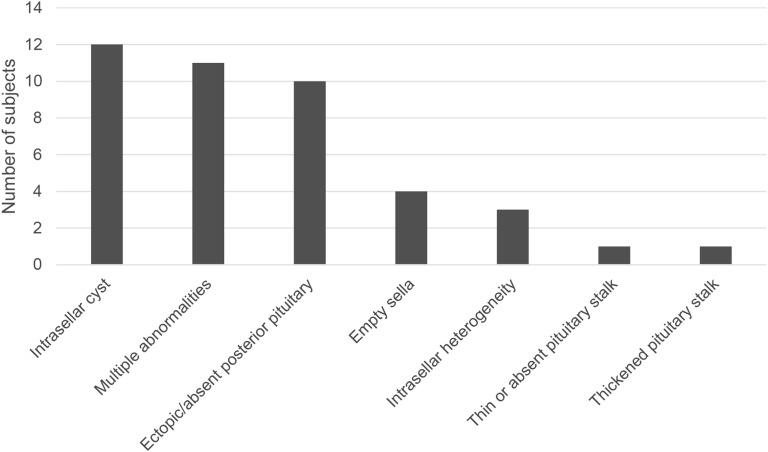
MRI results were available for 139 of the 171 subjects with GHD. No abnormality was seen in 97 subjects (70%), and the remaining 42 subjects had at least one abnormal finding. The frequency of the different MRI abnormalities is shown in Fig. 2. Of the seven subjects who developed MPHD, five had an abnormal MRI (71%), whereas MRI abnormalities were seen in only 37 of 131 subjects who did not develop MPHD (28%). Subjects with an MRI abnormality were more likely to develop MPHD than those without (OR, 6.3; 95% CI, 1.2 to 33.7).
Figure 2.
Frequency of MRI abnormalities.
C. Adverse Events
The rate of screening for biochemical adverse events (dysglycemia, renal injury, and liver function abnormalities) was investigated in the entire cohort (n = 328; both GHD and ISS/SHOX deficiency subjects).
Three subjects (0.9%) developed dysglycemia, as evidenced by HbA1c >6%. In two subjects, HbA1c peaked at 6.2%. One subject’s HbA1c normalized without intervention; in the other, HbA1c remained intermittently between 6.0% to 6.2%, interspersed with normal values. In both of these subjects, the body mass index was greater than the 97th percentile for age and sex. In the third subject, HbA1c peaked at 6.5% but fell to 6.0% with no intervention. There were no identifiable risk factors for the development of insulin resistance in this individual.
Fourteen subjects had transient elevations in serum creatinine levels 1 to 1.5 times the upper limit of normal. All elevations resolved spontaneously, with none persisting or progressing. No abnormalities in blood urea nitrogen levels or on urine dipstick analysis for hematuria or proteinuria were identified among these 14 subjects.
Elevated liver enzymes (AST, ALT) were seen in 39 of our 328 subjects (11.9%). None of the 39 subjects was found to have an elevated marker of bile duct injury (GGT, ALP) or abnormalities of synthetic liver function (hypoglycemia, hypoalbuminemia, hyperbilirubinemia). In 36 of the 39 subjects, the AST/ALT elevations were within 1.0 to 3.0 times the upper limit of normal, consistent with a grade 1 adverse event as per Common Terminology Criteria for Adverse Events guidelines [11]. Elevations were transient and resolved spontaneously in all but two subjects. In one of these subjects, the persistent elevations were thought to be related to nonalcoholic fatty liver disease due to comorbid obesity. In the other subject, a complete work-up was performed, including liver biopsy, with no underlying etiology found. A trial of GH did not result in resolution of the transaminitis. Three of the 39 subjects had elevated enzymes between 3.0 to 5.0 the upper limit of normal at some point during their course of GH treatment. The elevated enzymes resolved spontaneously in two of these subjects. In the third subject, liver enzymes normalized with the cessation of atomoxetine, a medication used to treat attention-deficit hyperactivity disorder, and with the institution of a gluten-free diet after a diagnosis of celiac disease was made.
There was no association between the underlying diagnosis (GHD vs ISS/SHOX deficiency) and the occurrence of any of the abnormalities identified in HbA1c, creatinine, or liver transaminase concentrations.
3. Discussion
There is growing recognition of the need to reduce unnecessary investigations and procedures in medicine. In 2012, the Choosing Wisely campaign became a clear example of this trend for more thoughtful use of evidence-based testing, and the campaign has spread across medical specialties and around the world [12]. The judicious and appropriate use of testing has clear benefits to patients and to the health care systems as a whole. When tests are done unnecessarily, false-positive results can lead to patient and family stress, to further unnecessary testing and diagnostic procedures, and to increased health care costs. In children treated with GH, routine laboratory screening for the development of MPHD and for adverse effects of GH treatment is regularly performed at our institution and at many other pediatric institutions. In light of the recognition of the disadvantages of over testing, this practice should be critically reviewed. The current study was performed to assess the utility of routine testing in otherwise well children on GH treatment with the goal of informing decisions around screening for MPHD and adverse events in this patient population.
MPHD developed in 4.1% of subjects in our GHD cohort. This percentage is far less than what has been previously reported by Tsai et al. [7] and Otto et al. [8]. This difference is likely due to the differing definitions used for pituitary hormone deficiencies, the length of follow-up, and the higher proportion of MRI abnormalities seen in the other cohorts. Furthermore, subjects in the latter cohort had a high rate of consanguinity (27%) and genetic etiologies (17%), which were excluded from our cohort. When looking solely at subjects with a normal MRI, our MPHD rate was 2.0%, which is similar to the rate of 3.8% seen by Tsai et al. [7] and similar to that found by the GeNeSIS cohort (2%) [6].
An abnormal MRI was the only variable that was associated with an increased risk of developing MPHD in our study. This finding was also seen in all three previously reported cohorts. Only two of our subjects with a normal MRI developed MPHD—one developing central hypothyroidism and the other hypogonadotropic hypogonadism. Although the early symptoms of hypothyroidism can be subtle, and thus there is a role in screening investigations to detect this diagnosis, hypogonadotropic hypogonadism is primarily detected clinically by the presence of delayed or stalled puberty. Thus, only 1% of subjects with a normal MRI developed an additional pituitary hormone deficiency (i.e., central hypothyroidism) that was perhaps not otherwise evident clinically. These results call into question the need for frequent routine pituitary screening in otherwise well children with isolated GHD and a normal MRI. These results may not apply to those with comorbid autoimmune chronic illness because we excluded these subjects from our analysis. Future research is needed to investigate if these patients are at higher risk of developing MPHD due to autoimmune hypophysitis, for example, and a more conservative approach to screening in this population would be prudent in the meantime.
Although we did not see any cases of ACTH deficiency or central diabetes insipidus, these disorders have been noted in the other cohorts. Similarly to hypogonadotropic hypogonadism, central diabetes insipidus presents clinically with a history of polyuria with or without polydipsia, and thus routine biochemical screening for this hormone deficiency, in the absence of symptoms, is likely unwarranted. ACTH deficiency, on the other hand, may present acutely as an adrenal crisis without preceding clinical clues, providing a rationale for routine screening. However, we identified no cases of ACTH deficiency, and the larger GeNeSIS study reported only three subjects (0.05% of the total) who developed ACTH deficiency. Some subjects in the GeNeSIS cohort did not undergo MRI, so it is possible that this number might be even lower if one only considers those with a normal MRI. Our findings, as well as those of the GeNeSIS study, call into question the role for routine testing for ACTH deficiency for patients with a normal MRI; however, because missing this diagnosis can have substantial clinical consequences, the role of routine biochemical screening for ACTH deficiency is more debatable than the routine screening for other disorders.
Subjects in our full cohort were also screened for the development of insulin resistance, renal injury, and abnormal liver function. No subject developed frank diabetes mellitus; three subjects developed an elevated HbA1c, but two of these three subjects had underlying risk factors. This finding is consistent with the Pediatric Endocrine Society’s GH treatment guidelines that glucose metabolism screening should be undertaken in patients at increased risk for diabetes [2]. Many subjects had transient elevations in serum creatinine, but none developed permanent kidney injury. This is consistent with other studies looking at the impact of GH on renal function [13, 14]. We found that many subjects had transiently elevated liver enzymes; however, only two subjects had persistent elevations, and in neither case were the laboratory abnormalities thought to be related to GH treatment, similar to other studies [15]. Thus, despite the large number of laboratory tests that were done, only one patient had an abnormality (elevated HbA1c) that was not suggested based on history and clinical findings and was thought to be secondary to GH use. One could further posit that not only did none of this testing find anything too meaningful, it likely led to even more unnecessary testing. The finding of an abnormal laboratory value, even if nonsignificant, often leads to further testing to track the course of the abnormality and to look for an etiology.
Our study is limited by a relatively short length of average follow-up, although one-third of our subjects were followed for at least 5 years. Other studies that have followed children with GHD for longer periods of time found a higher rate of MPHD, although at a significantly lower frequency in those with a normal vs abnormal MRI, re-emphasizing that the indications for routine pituitary hormone screening in those with normal vs abnormal MRI are likely different. Although routine biochemical screening was generally conducted within our clinic once a year, there was some variation in testing frequency by the physician. This could have led to an underdiagnosis of abnormalities. It is important therefore that our results are corroborated in future cohorts. The selection of which patients would undergo GH stimulation testing in our clinic was also physician dependent, but this test is required by our center for the diagnosis of GHD. We acknowledge there is controversy surrounding the use of GH stimulation testing to make the diagnosis of GHD and that this testing may have led to some patients with normal somatotropic reserve being included in the GHD group [16]. Inclusion of individuals with normal reserve (who might have a lower likelihood of developing other pituitary defects) within the GHD group could have skewed our data toward a lower rate of additional pituitary defects being diagnosed over time within the GHD group. However, against this possibility is the finding of lower IGF-1 values in the GHD vs ISS group (Table 1). Moreover, given that this is the method used in our center and by many other pediatric endocrinologists, we felt that using it to define GHD in our cohort would allow our results to be generalizable. Despite these limitations of a retrospective analysis, our study has several strengths. Our definition of hormone deficiencies was clinically meaningful. As well, our cohort represents all patients treated with GH at our institution in the time frame studied, thus reducing possible reporting and selection bias.
In summary, the higher frequency of MPHD in patients with MRI abnormalities confirms that these children warrant routine pituitary hormone laboratory screening for central hypothyroidism and likely ACTH deficiency as well. However, our finding of a low rate of MPHD among individuals without clinical risk factors or findings calls into question the need for routine biochemical screening in children with isolated idiopathic GHD, normal MRIs, and no clinical indications. If our results are corroborated, a more prudent approach for this group may be targeted testing based on findings of the history and physical examination. Similarly, regarding laboratory screening for adverse events, our results suggest, consistent with the Pediatric Endocrine Guidelines [2], that one should consider limiting testing for hyperglycemia or diabetes to those with predisposing risk factors, such as obesity, Turner syndrome, or a history of malignancy. There does not appear to be a role for routine testing of liver or renal function. Routine laboratory screening for otherwise well children with isolated GHD on GH treatment may be an area for pediatric endocrinologists to begin “choosing wisely.”
Acknowledgments
Disclosure Summary: The authors have nothing to disclose.
Glossary
Abbreviations:
- GeNeSIS
Genetics and Neuroendocrinology of Short Stature International Study
- GH
growth hormone
- GHD
growth hormone deficiency
- ISS
idiopathic short stature
- MPHD
multiple pituitary hormone deficiencies
References and Notes
- 1. Child CJ, Blum WF, Deal C, Zimmermann AG, Quigley CA, Drop SL, Cutler GB Jr, Rosenfeld RG. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with isolated growth hormone deficiency due to organic causes. Eur J Endocrinol. 2016;174(5):669–679. [DOI] [PubMed] [Google Scholar]
- 2. Grimberg A, DiVall SA, Polychronakos C, Allen DB, Cohen LE, Quintos JB, Rossi WC, Feudtner C, Murad MH; Drug and Therapeutics Committee and Ethics Committee of the Pediatric Endocrine Society. Guidelines for growth hormone and insulin-like growth factor-I treatment in children and adolescents: growth hormone deficiency, idiopathic short stature, and primary insulin-like growth factor-I deficiency. Horm Res Paediatr. 2016;86(6):361–397. [DOI] [PubMed] [Google Scholar]
- 3. Cutfield WS, Wilton P, Bennmarker H, Albertsson-Wikland K, Chatelain P, Ranke MB, Price DA. Incidence of diabetes mellitus and impaired glucose tolerance in children and adolescents receiving growth-hormone treatment. Lancet. 2000;355(9204):610–613. [DOI] [PubMed] [Google Scholar]
- 4. Child CJ, Zimmermann AG, Scott RS, Cutler GB Jr, Battelino T, Blum WF; GeNeSIS International Advisory Board. Prevalence and incidence of diabetes mellitus in GH-treated children and adolescents: analysis from the GeNeSIS observational research program. J Clin Endocrinol Metab. 2011;96(6):E1025–E1034. [DOI] [PubMed] [Google Scholar]
- 5. Bell J, Parker KL, Swinford RD, Hoffman AR, Maneatis T, Lippe B. Long-term safety of recombinant human growth hormone in children. J Clin Endocrinol Metab. 2010;95(1):167–177. [DOI] [PubMed] [Google Scholar]
- 6. Blum WF, Deal C, Zimmermann AG, Shavrikova EP, Child CJ, Quigley CA, Drop SL, Cutler GB Jr, Rosenfeld RG. Development of additional pituitary hormone deficiencies in pediatric patients originally diagnosed with idiopathic isolated GH deficiency. Eur J Endocrinol. 2013;170(1):13–21. [DOI] [PubMed] [Google Scholar]
- 7. Tsai SL, Laffan E, Lawrence S. A retrospective review of pituitary MRI findings in children on growth hormone therapy. Pediatr Radiol. 2012;42(7):799–804. [DOI] [PubMed] [Google Scholar]
- 8. Otto AP, França MM, Correa FA, Costalonga EF, Leite CC, Mendonca BB, Arnhold IJ, Carvalho LR, Jorge AA. Frequent development of combined pituitary hormone deficiency in patients initially diagnosed as isolated growth hormone deficiency: a long term follow-up of patients from a single center. Pituitary. 2015;18(4):561–567. [DOI] [PubMed] [Google Scholar]
- 9. Kelly A, Winer KK, Kalkwarf H, Oberfield SE, Lappe J, Gilsanz V, Zemel BS. Age-based reference ranges for annual height velocity in US children. J Clin Endocrinol Metab. 2014;99(6):2104–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Greulich WPS, ed. Radiographic Atlas of Skeletal Development of the Hand and Wrist. 2nd edStanford, CA: Stanford University Press; 1959. 10.1097/00000441-195909000-00030. [DOI] [Google Scholar]
- 11. National Cancer Institute. Common terminology criteria for adverse events. Bethesda, MD: National Institutes of Health; 2009:4. [Google Scholar]
- 12. Levinson W, Kallewaard M, Bhatia RS, Wolfson D, Shortt S, Kerr EA; Choosing Wisely International Working Group. ‘Choosing Wisely’: a growing international campaign. BMJ Qual Saf. 2015;24(2):167–174. [DOI] [PubMed] [Google Scholar]
- 13. Mehls O, Lindberg A, Haffner D, Schaefer F, Wühl E, German KB, Group ET German KIGS BoardESCAPE Trial Group. Long-term growth hormone treatment in short children with CKD does not accelerate decline of renal function: results from the KIGS registry and ESCAPE trial. Pediatr Nephrol. 2015;30(12):2145–2151. [DOI] [PubMed] [Google Scholar]
- 14. Hodson EM, Willis NS, Craig JC. Growth hormone for children with chronic kidney disease. Cochrane Database Syst Rev. 2012; (2):CD003264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Salerno M, Di Maio S, Ferri P, Lettiero T, Di Maria F, Vajro P. Liver abnormalities during growth hormone treatment. J Pediatr Gastroenterol Nutr. 2000;31(2):149–151. [DOI] [PubMed] [Google Scholar]
- 16. Murray PG, Dattani MT, Clayton PE. Controversies in the diagnosis and management of growth hormone deficiency in childhood and adolescence. Arch Dis Child. 2016;101(1):96–100. [DOI] [PubMed] [Google Scholar]