Figure 4.
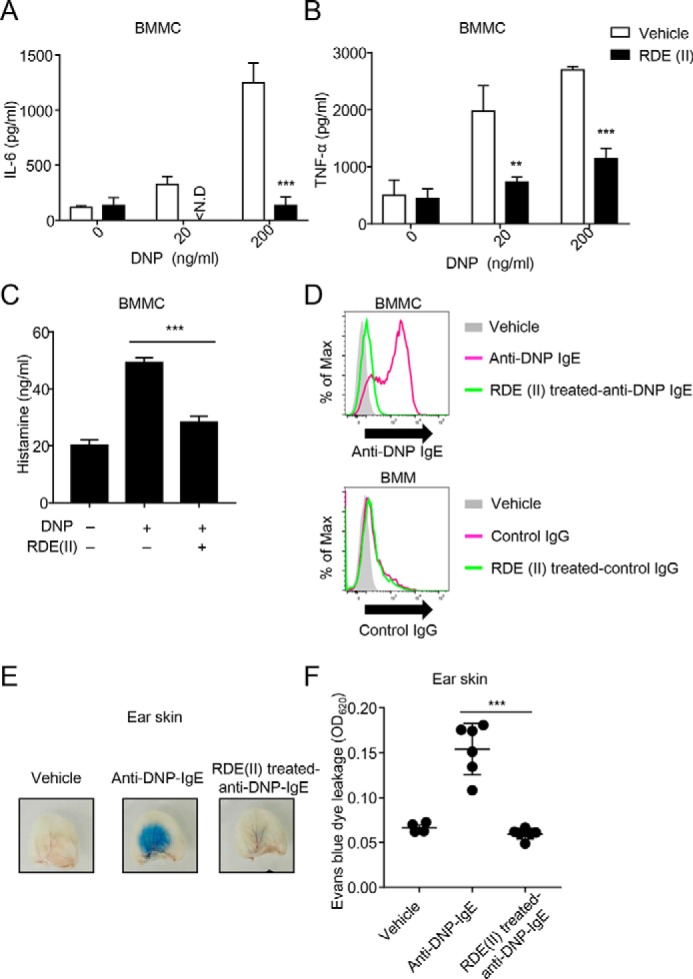
RDE (II)–treated IgE cannot induce anaphylaxis. A and B, effects of RDE (II)–treated IgE reduced the expression levels of IL-6 (A) or TNFα (B) from BMMCs. Two μg/ml anti-DNP IgE was treated with RDE (II) overnight (12–20 h), followed by incubation at 56 °C. Then, the antibodies sensed BMMC for 2 h, followed by incubation with HSA–DNP as indicated overnight (12–20 h). Then, the supernatant was collected and analyzed with quantitative ELISA for IL-6 (A) or TNFα (B). **, p < 0.01, and ***, p < 0.001 (Student's t test). N.D., not detected. C, 2 μg/ml anti-DNP IgE was treated with RDE (II) overnight (12–20 h). BMMCs were sensed overnight (12–20 h) and then incubated with 200 ng/ml HSA–DNP for 1 h. The supernatant was then obtained and analyzed with competitive ELISA for histamine. ***, p < 0.001 (one-way ANOVA). D, 20 μg/ml biotin-conjugated anti-DNP IgE or control IgG (MOPC-21) was treated with RDE (II) overnight (12–20 h). BMMCs or BMMs were incubated with the antibodies for 30 min, followed by incubation with APC-conjugated streptavidin. The binding level was analyzed by FACSCantoII. E, anti-DNP IgE was treated with RDE (II) overnight (12–20 h). The ears of mice were passively sensitized with 20 ng of RDE (II)–treated anti-DNP IgE. One day later (18–24 h), all mice were intravenously injected with 200 μg of DNP–HSA containing Evans blue dye. Thirty minutes later, the ears were obtained and observed in the transudation of Evans blue dye to determine vascular permeability. F, the absorbance of the extraction from the ear was measured at 620 nm. ***, p < 0.001 (one-way ANOVA). All data are representative of at least two independent experiments and indicate the mean ± S.D.
