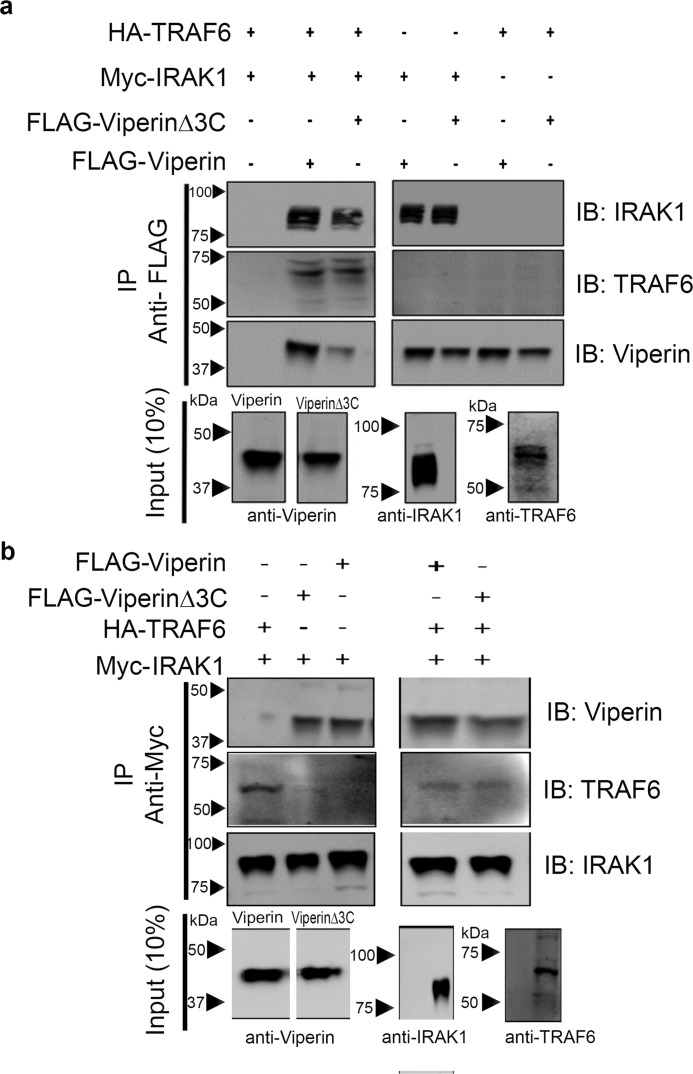
Figure 3.
IRAK1 mediates formation of the complex between viperin, IRAK1, and TRAF6. Immunotagged genes were transfected into HEK 293T cells and cell extracts were prepared 30 h post-transfection. FLAG-viperin or FLAG-viperinΔ3C cell extracts were mixed with Myc-IRAK1 and TRAF6 cell extracts in a ratio of 1:1:1. Proteins were immunoprecipitated with either anti-FLAG (viperin) or anti-Myc (IRAK1) antibodies and analyzed by immunoblotting (IB) with the indicated antibodies. Control experiments confirmed the specificity of the antibodies used for immunoprecipitation (IP), see Fig. S5. a, immunoprecipitation of viperin indicates that viperin binds IRAK1 but not TRAF6. b, immunoprecipitation of IRAK1 indicates that IRAK1 binds both viperin and TRAF6. Mutations in the radical SAM domain (viperinΔ3C) do not affect the ability of viperin to interact with IRAK1. Representative blots are shown from two independent experiments. Cytosolic extracts were also immunoblotted to confirm expression levels of each individual protein of interest (10% input). For details see, “Experimental procedures.”